With most techniques for 3D cell culture being laborious and expensive, researchers have presented a low-cost and effective nanofibrous scaffold for the spontaneous formation of 3D breast cancer microtissue within the journal ACS Omega.

Study: Spontaneous Formation of 3D Breast Cancer Tissues on Electrospun Chitosan/Poly(ethylene oxide) Nanofibrous Scaffolds. Image Credit: luchschenF/Shutterstock.com
In a bid to advance cancer testing protocols, the creation of in vitro three-dimensional cell cultures may be more valuable than its two-dimensional counterpart to understand the biology of cancer in a simulated environment most like that of in vivo experimentation.
Why Conventional Cell Culture May Not Be Enough?
In vitro 2D cell culture holds a significant role in understanding the microenvironment and cellular interactions with advantages such as improved environmental control cell observation.
However, while this approach tends to be one of the first steps to comprehending the behavior of cells within certain conditions and parameters, or even as a proof-of-concept to conceptualize the interactions within the body, it has limitations that cannot be ignored.
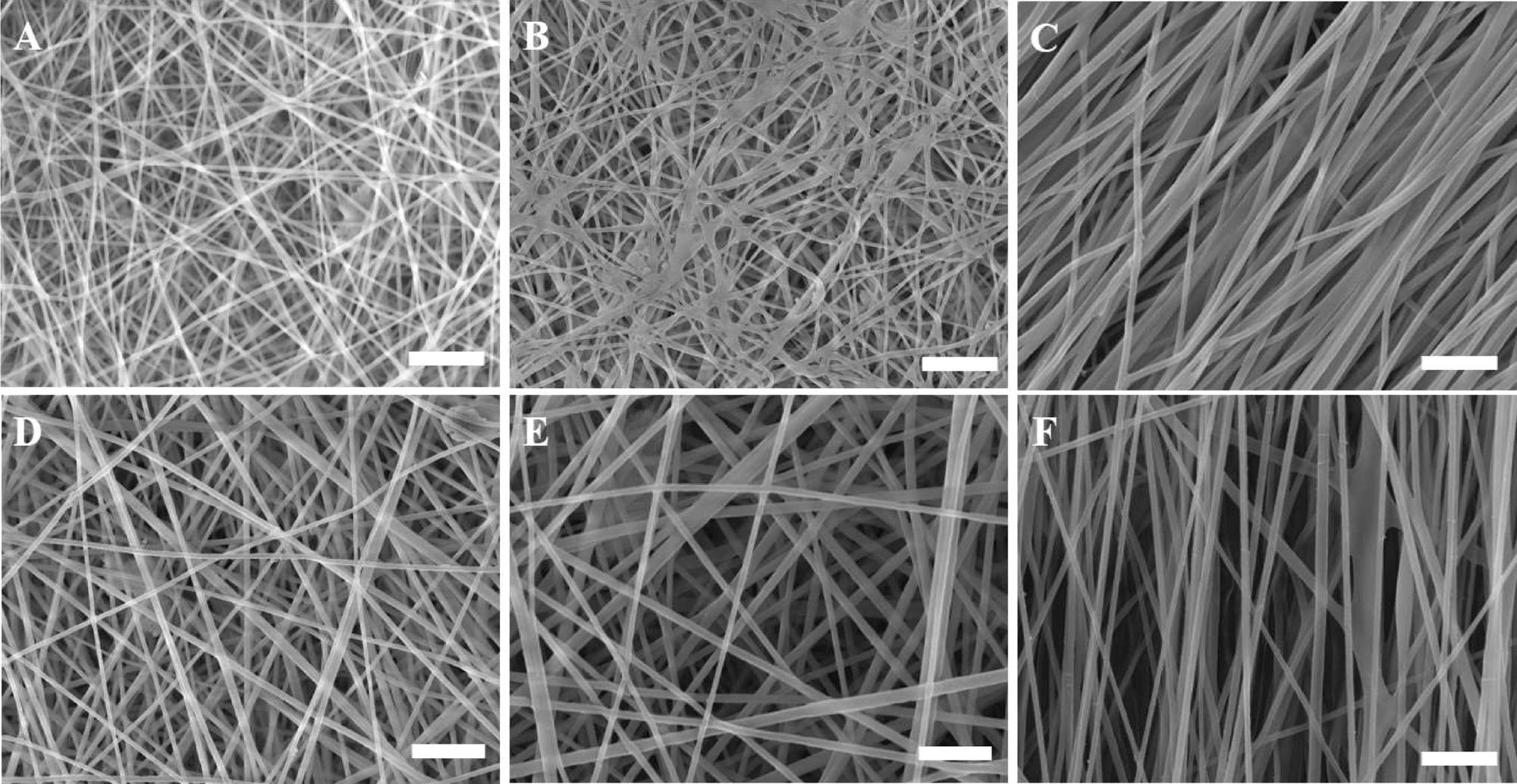
SEM micrographs of non-aligned (random) and aligned electrospun nanofibrous scaffolds showing the effect of various electrospinning processing parameters on the fiber diameter of the scaffolds. (A) R-R1-C2P1, (B) R-R4-C2P1, (C) A-R4-C2P1, (D) R-R1-C4P1, (E) R-R4-C4P1, and (F) A-R4-C4P1. The scale bar is 1 μm. © Rabie, A., et al (2022)
These limitations can consist of being unable to provide the same microenvironment as that within the body. This may include being unable to be affected by altered gene expression, compatibility within an in vivo system and even personalized drug sensitivity. The environment within the body with intricate biological systems is very complex. While in vitro 2D cell culture can provide decent insight, it cannot be fully comparable to the native biological system within the body.
The drawbacks of 2D cell culture illustrate why 3D cell culture may be more useful when attempting to understand and study cells and tissue more closely outside of the body.
3D cell culture provides a higher degree of structural complexity and homeostasis comparable to tissues and organs and allows for a better understanding of cells within a more natural environment.
Additionally, this type of cytoarchitecture can be used to simulate a natural environment to study significant tumor characteristics, including hypoxia, anti-apoptotic behavior, and dormancy.
However, while 3D cell culture can provide a more fulfilling understanding of cell behavior, it can also hold disadvantages including being expensive, laborious with many steps, such as separation of single cells from spheroid structures through proteolytic degradation which can be time-consuming and take between several hours to days to complete.
It may also be difficult to repeat with the same results depending on the 3D model chosen as well as lack sustainability.
Innovative Nanofibrous Scaffold for 3D Microtissue Formation
With drawbacks of 3D tissue culture, including high expense and laborious steps, these innovative researchers have presented a low-cost and highly effective method that could provide an alternative.
The researchers utilized a nanofibrous scaffold for the spontaneous formation of 3D breast cancer microtissues. This approach consisted of electrospun aligned and non-aligned scaffolds which were prepared with two different types of polymers, chitosan and polyethylene oxide, with two different chitosan concentrations, 2 and 4 wt %.
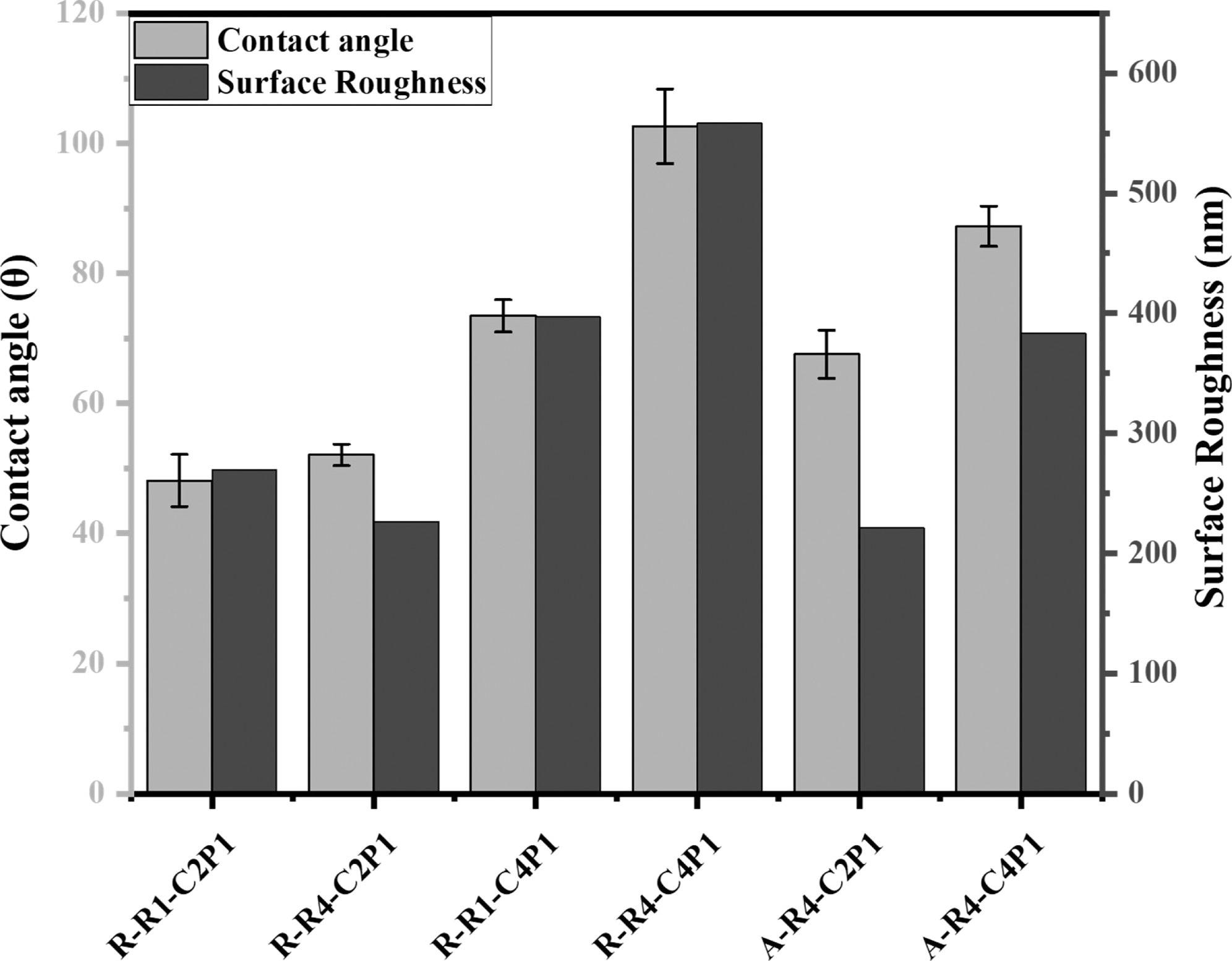
Wettability and surface roughness of non-aligned and aligned electrospun nanofibrous scaffolds. The figure describes the effect of the CS concentration and pump flow rate on the surface wettability and roughness of the prepared scaffolds. © Rabie, A., et al (2022)
Chitosan, a polysaccharide polymer derived from chitin, is a very interesting and useful material that has been utilized in different applications due to its biodegradable, antibacterial and environmentally friendly characteristics. Additionally, with its hydrophobic surface chemistry, chitosan has the ability to facilitate cell adhesion which would be beneficial for a 3D cell culture model.
This proof-of-concept of utilizing a nanofibrous scaffold to culture breast cancer cells has been previously investigated within existing literature, with MCF-7 cells being cultured on polycaprolactone nanofibrous scaffold increasing cancer stem cell marker expression.
The innovative researchers of this study have utilized different parameters using chitosan within two different concentrations to create an optimized scaffold. This would allow the study of surface nanotopography such as pore size and flow, as well as their effect on enabling the formation of breast cancer microtissue using human breast cancer cells, MCF-7.
Future Implications
This innovative approach to 3D cell culture is simple, low-cost and highly effective, with the ability to sustain cell viability and active cell growth for at least 17 days.
The fabrication process is formed through non-toxic components, which are FDA approved – a potentially viable method of 3D cell culture that can aid with providing cancer cells a natural microenvironment.
The environmentally friendly aspect of this approach with the use of chitosan allows this 3D model to be more sustainable without the inclusion of hazardous chemicals. Additionally, this 3D chitosan/polyethylene oxide nanofibrous scaffold could be utilized for drug screening to develop cancer treatments comparable to in vivo biological systems.
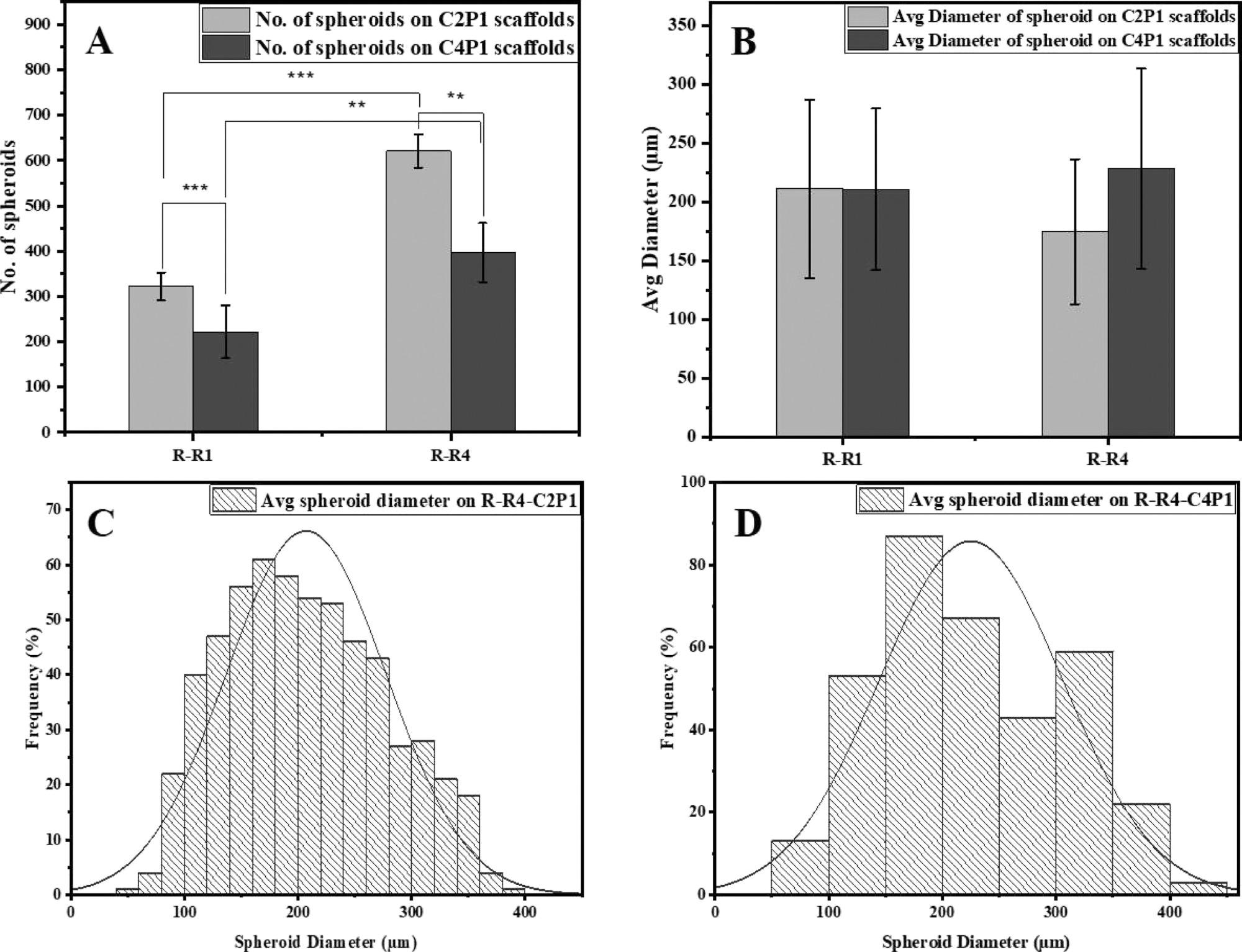
Effects of scaffold surface topography on MCF-7 spheroid formation. (A) Number of MCF-7 spheroids formed on R-R1 and R-R4 for the C2P1 and C4P1 scaffolds after 7 days of culturing. (B) Average spheroid diameter on R-R1 and R-R4 for the C2P1 and C4P1 scaffolds. (C,D) Histograms show spheroid size distribution on R-R4 of C2P1 and C4P1 scaffolds, respectively. © Rabie, A., et al (2022)
Having a more similar microenvironment to the body than 2D cell culture could enhance the efficacy of cancer therapeutics, and with reproducible results, this method can prove to be more useful than conventional cell culture approaches.
Continue reading: Disease Diagnosis with Magnetic Nanoparticles
Reference
Rabie, A., Ali, A., Al-Zeer, M., Barhoum, A., EL-Hallouty, S., Shousha, W., Berg, J., Kurreck, J. and Khalil, A., (2022) Spontaneous Formation of 3D Breast Cancer Tissues on Electrospun Chitosan/Poly(ethylene oxide) Nanofibrous Scaffolds. ACS Omega,. Available at: https://pubs.acs.org/doi/10.1021/acsomega.1c05646
Further Reading
Kapałczyńska, M., Kolenda, T., Przybyła, W., Zajączkowska, M., Teresiak, A., Filas, V., Ibbs, M., Bliźniak, R., Łuczewski, Ł. and Lamperska, K., (2016). 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Archives of Medical Science,. Available at: https://doi.org/10.5114/aoms.2016.63743
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.