The latest article published in the journal materials focuses on a new method of carbon dioxide (CO2) adsorption upon magnesium oxide (MgO) with the hope of improving carbon capture systems.

Study: Morphology Design and Fabrication of Bio-Inspired Nano-MgO–Mg(OH)2 via Vapor Steaming to Enable Bulk CO2 Diffusion and Capture. Image Credit: Dmitry Kovalchuk/Shutterstock.com
Nanomaterials and Carbon Dioxide
The rising level of carbon dioxide (CO2) in the air, which leads to globally changing ecosystems, has stimulated novel designs and methods to mitigate its effects.
Owing to rising build-up in the atmosphere, CO2 is one of the most significant human pollutants. As a result, considerable efforts must be made to lower CO2 levels through the use of modern and effective CO2 collection technology.
Carbon dioxide capture and storage (CCS) technology might play a significant role in this regard. Many efforts have been made to develop efficient CO2 collection materials.
Utilization of Magnesium Oxide for CO2 Adsorption
Eco-friendly nanoparticles have lately become critical in a wide range of technology development fields. Magnesium oxide (MgO) and magnesium hydroxide (Mg (OH)2) are the most viable choices in terms of being ecologically friendly.
MgO is extensively employed for industrial purposes owing to intrinsic properties such as heat resistance, insulating characteristics, and tensile stability.
CO2 fillers based on MgO have been discovered as effective at ambient temperature. As a result, several researchers have investigated the use of magnesium oxide to capture CO2.
What are the Morphological Advantages of Magnesium Hydroxide?
Mg (OH)2 has a surprising spectrum of crystalline forms, including spindles, tubing, fibers, granules, rod, and even florals or canyons, among many others.
Absorption of a magnesium compound with an alkaline medium, the sol-gel methodology, microwave-assisted chemical biosynthesis, thermal oxidation, and ammonia gas bubbling furnaces are all methods for creating Mg (OH)2.
The MgO hydration method, on the other hand, is considered to be among the best cost-effective approaches to date. The hydration process is influenced by various parameters, including MgO properties, externally applied microenvironment, hydration warmth, and commencement location.
As a result, to synthesize Mg (OH)2 with the necessary properties, the MgO hydration reaction parameters must be carefully managed.
Physiochemical Proceedings of Carbon Dioxide Adsorption
CO2 adsorption by MgO leads to the production of an exterior MgCO3 layer, which prevents carbon dioxide from penetrating the central MgO sections via the MgCO3 casing. As a result, the CO2 collecting potential is significantly lower than theoretically predicted.
MgO–Mg (OH)2 composites contain an intertwined CO2 capture area characterized by MgO and a CO2 dispersion zone controlled by MgO/Mg (OH)2 junctions, according to this recent research.
CO2 mechanically collected by MgO can penetrate the innermost layer and be biochemically collected by the inner MgO. Furthermore, H2O vapor may prove to be a further supportive mechanism for CO2 absorption.
Carbon dioxide Capture Capacity of Samples
For 1.5 hours, specimens weighing 5 to 7 mg were examined at 30 °C with a continuous stream of highly pure CO2.
When the testing time reached 1.5 h, absorption attained its highest CO2 capture capability.
The team found that carbon dioxide capture was greater when specimens were submitted to steam than when specimens were not subjected to steam (no-steam sample). The CO2 capability of the no-steam specimen was 2.43 wt.%, compared to 4.12 wt.% for the sample exposed to steam for 20 minutes.
After 20 minutes of steam exposure, the capture capacity significantly quadrupled when contrasted to the no-steam specimen. Mg (OH)2 synthesis may enhance contact area, enabling for more CO2 capture.
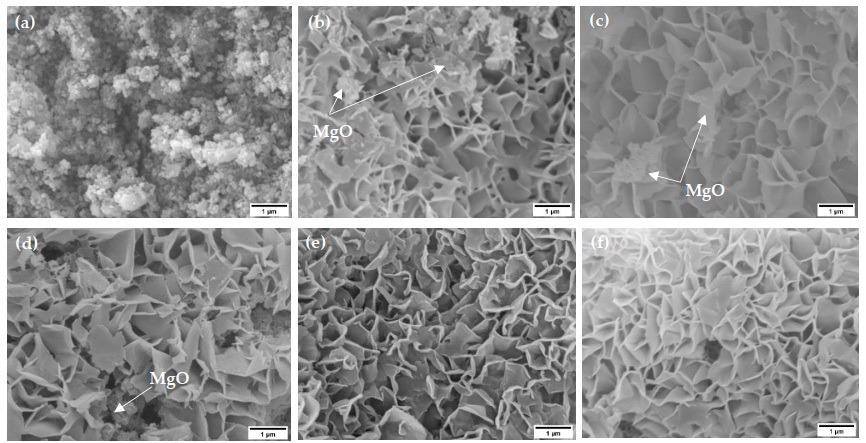
SEM images of electrospun MgO samples (a) before exposure to steam (no-steam) and after exposure to steam for (b) 2-min, (c) 5-min, (d) 10-min, (e) 15-min, and (f) 20-min at 100 °C. © Senevirathna, H.L., Wu, S., Lee, W.P.C., Wu, P. (2022)
Before steam treatment (no-steam), the specimen microstructure revealed structured nanoparticle and sheet-like formations.
Flower-like formations might be seen after steaming contact. After varying timeframes of steaming treatment, interwoven perpendicular nano-sheets of Mg (OH)2 with a mean size of 100 nm were strongly and evenly formed on the specimens.
To summarize, the team investigated the morphological control of MgO–Mg (OH)2 composite materials for efficient CO2 adsorption at 30 °C. The test data reveal that by heating the specimen for 20 minutes, the CO2 sequestration at 30 °C was enhanced from 2.43 wt.% to an astounding 4.12 wt. %.
This is related to the generation of Mg (OH)2, where the carbonate production and dissolution mechanisms were regulated and optimized in the study.
Continue reading: Laying the Groundwork for Metal Organic Framework Carbon Capture.
Reference
Senevirathna, H.L., Wu, S., Lee, W.P.C., Wu, P. (2022) Morphology Design and Fabrication of Bio-Inspired Nano-MgO–Mg (OH)2 via Vapor Steaming to Enable Bulk CO2 Diffusion and Capture. Materials. 15(2). 680. Available at: https://www.mdpi.com/1996-1944/15/2/680
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.