In a recent study published in the journal Nanomaterials, researchers analyzed the effectiveness of pristine reduced graphene oxide (rGO) in removing cationic pollutants such as methylene blue (MB) and mercury-(II) (Hg-(II)) from contaminated water.

Study: Cationic Pollutant Removal from Aqueous Solution Using Reduced Graphene Oxide. Image Credit: kosmos111/Shutterstock.com
The pristine rGO showed an adsorption capacity of 121.95 mg g−1 and 109.49 mg g−1 with an efficiency of about 89% and 73% at 298 K for MB and Hg (II), respectively. Additionally, the captured cationic pollutants reacted to both physisorption and chemisorption simultaneously.
Cationic Water Pollutants
Industrial and agricultural wastes such as dyes and heavy metals are a significant portion of cationic water pollutants.
These are harmful to aquatic organisms and humans as they are carcinogenic, mutagenic, and promote dysfunction of important human organs such as the kidney, liver, reproductive system, and central nervous system.
The methods to remove these cationic pollutants include membrane filtration, ion exchange, adsorption, electrocoagulation, electroflotation, and electrodeposition.
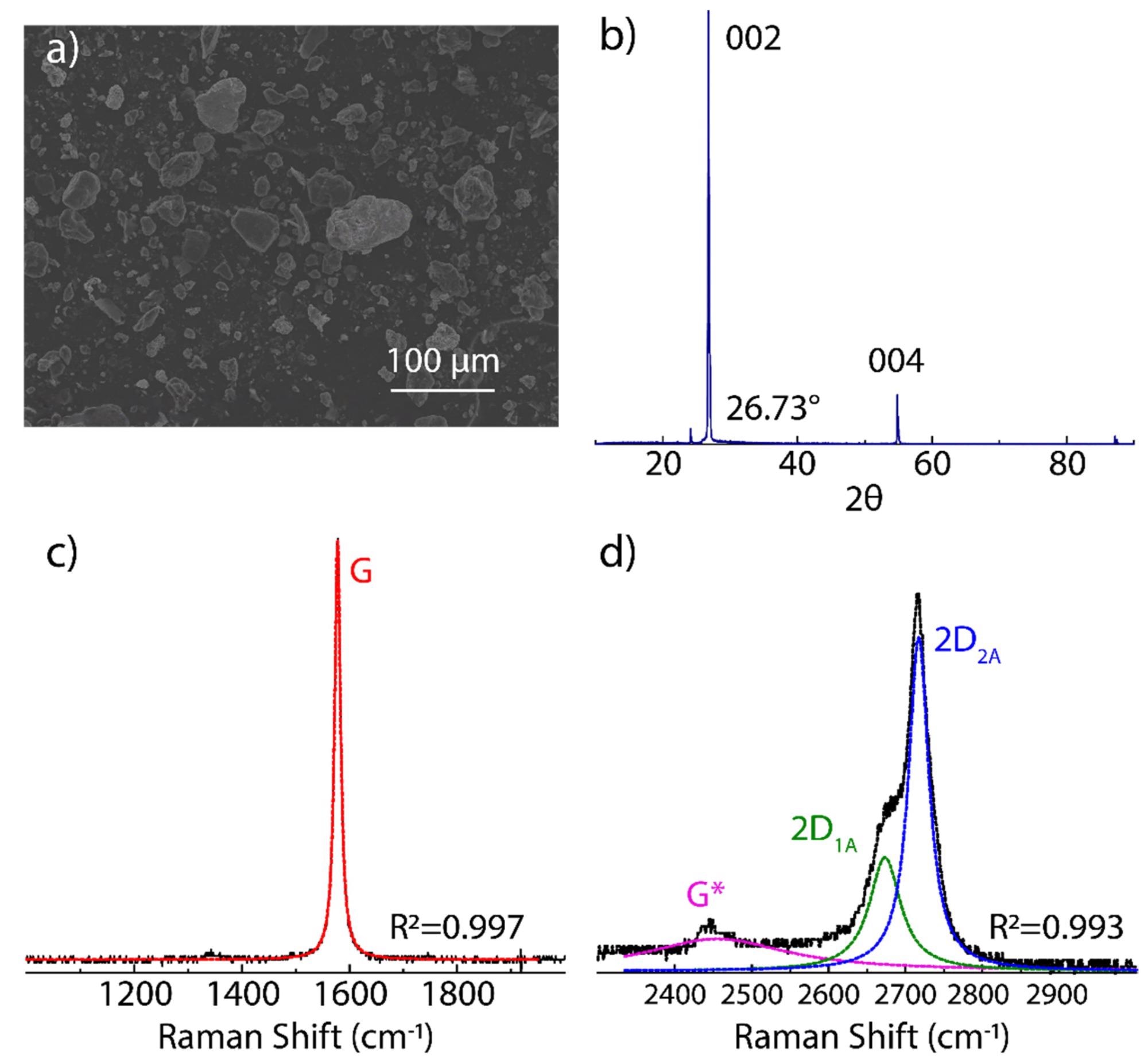
Figure 1. Characterization of starting graphite source: (a) SEM morphology, (b) XRD measurement, and (c,d) Raman spectrum from 1000 to 3000 cm−1 recorded using 532 excitation laser. The intensity was normalized by the most intense peak. The Raman spectrum was fitted using Lorentzian functions. © Tene, T., et al (2022)
Among these, the absorption method is more cost-effective and environmentally friendly, which uses activated carbon. However, the high cost and the complicated regeneration process limit the large-scale applications of activated carbon.
As an alternative, rGO has garnered much attention due to its hydrophilic and semiconducting properties. Moreover, the presence of oxygen functional groups on the surface of rGO allows strong covalent bonding with chelating groups.
These chelating groups react with metal ions to form a stable water-soluble complex. Hence, heavy metal ions can be effectively removed from water through adsorption without the requirement of any additional energy sources.
About the Study
In this study, the researchers used the eco-friendly oxidation-reduction method to synthesize rGO from pure graphite in a step-wise manner. Subsequently, the absorption efficiencies of pristine rGO and extra functionalized rGO were measured and compared in the prepared MB and Hg (II) solutions at the room temperature of 298K for 30 min and 20 min, respectively.
In the oxidation process, pure graphite powder was mixed with sulfuric acid (H2SO4) and potassium permanganate (KMnO4) to cause an exothermic oxidation reaction followed by removal of residual compounds using hydrogen peroxide (H2O2), hydrochloric acid (HCl), and distilled water to obtain pristine graphite oxide (GO) flakes.
The prepared GO flakes were centrifuged with citric acid and distilled water, then dried at 353 K for 2 hours to obtain pristine rGO powder. In extra functionalized rGO, oxygen functional groups rGO were functionalized with additional electroactive groups.
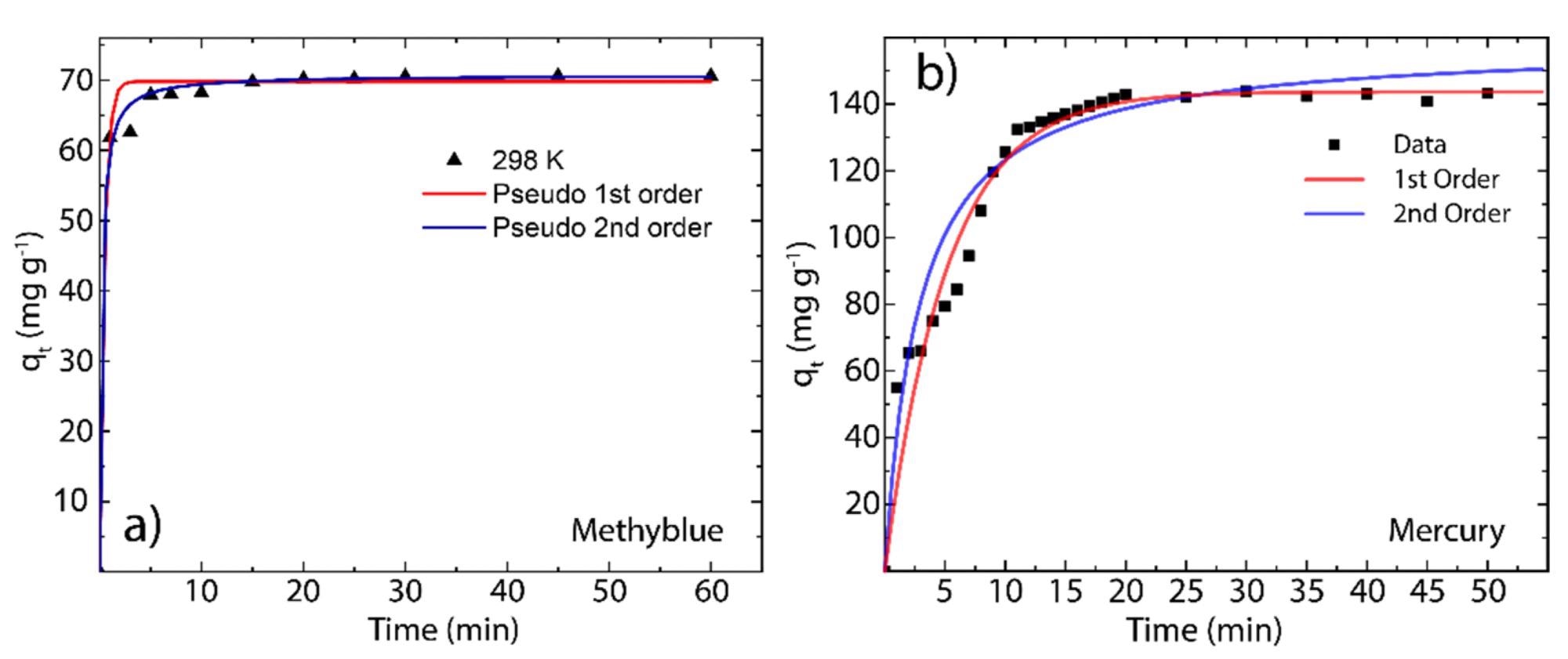
Figure 2. Adsorption kinetics of (a) MB on rGO and (b) Hg(II) on rGO as a function of contact time (60 min) at 298 K. © Tene, T., et al (2022)
Observations
Scanning electron microscopy (SEM) micrographs of GO revealed a face-to-face stacking of flakes and wrinkled aggregated flakes. In contrast, rGO had randomly organized flakes with surface morphology comprising mesopores and micropores.
Raman spectra of GO showed a highly broadened and very-low intense 2D peak compared to pure graphite. The FTIR spectrum of GO showed the presence of C-O-C bond at 1044 cm−1, C-O bond at 1222 cm−1, C=C bond at 1644 cm−1, C=O at 1729 cm−1, and C-H bond at 3426 cm−1. However, after the reduction, the rGO spectrum showed attenuated and weakened peaks of the above bonds due to removing oxygen-containing functional groups.
Furthermore, the adsorption kinetics of MB and Hg (II) onto rGO at 298K revealed that rGO rapidly captured MB molecules after 30 min, whereas for Hg (II) molecules, the equilibrium time of adsorption was 20 min.
The improved effectiveness of rGO in adsorbing cationic pollutants was due to the increase in recovered surface area and the presence of oxygen functional groups after the reduction process.
When the temperature of the solutions was increased, the maximum adsorption capacity of MB on rGO decreased from 121.95 to 107.53 mg g−1. For Hg (II) on rGO, adsorption capacity increased from 109.49 to 255.04 mg g−1, evidencing the significant influence of temperature on cationic adsorption on rGO.
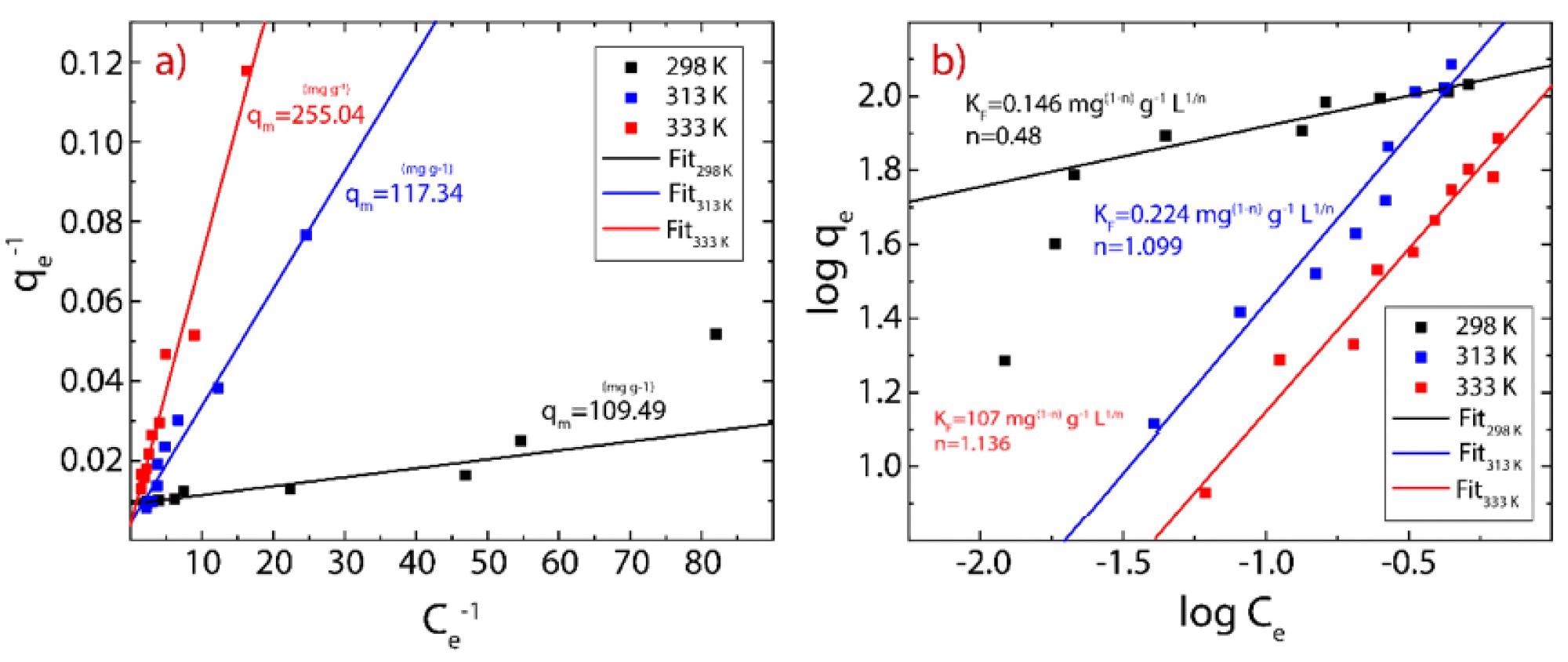
Figure 3. Adsorption isotherm of Hg(II) on rGO considering three different temperatures (289-333 K). (a) Langmuir model and (b) Freundlich model. © Tene, T., et al (2022)
Conclusions
In summary, the researchers investigated the effect of pristine rGO in the removal of cationic pollutants such as MB and Hg (II) from water. Also, the team used an eco-friendly oxidation-reduction process for the synthesis of rGO.
The obtained maximum adsorption capacity of both MB and Hg (II) were 121.95 and 109.49 mg g−1at 298 K, respectively, which were much higher than previously reported studies.
The achieved adsorption efficiencies of 89% and 73% at 298 K for MB and Hg (II) using pristine rGO, instead of extra functionalized rGO, provide a potential green alternative for wastewater decontamination.
Continue reading: The Future of Removing Microplastics from Water with Nanotubes.
Reference
Tene, T., Bellucci, S., Guevara, M., Viteri, E., Arias Polanco, M., Salguero, O., Vera-Guzmán, E., Valladares, S., Scarcello, A., Alessandro, F., Caputi, L., Vacacela Gomez, C., (2022) Cationic Pollutant Removal from Aqueous Solution Using Reduced Graphene Oxide. Nanomaterials,12, 309. https://www.mdpi.com/2079-4991/12/3/30
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.