Researchers from Indonesia and Norway have published an article in the journal polymers in which Polyvinyl alcohol is used as a foundation for carbon-nano fiber synthesis with the addition of silver nitrate.

Study: Carbon-Nano Fibers Yield Improvement with Iodinated Electrospun PVA/Silver Nanoparticle as Precursor via One-Step Synthesis at Low Temperature. Image Credit: whitehoune/Shutterstock.com
Introduction to Carbon Nanofibers
Carbon nanofibers (CNF) are appealing for use as reinforcement in polymer materials in engineering fields, supercapacitors, high-temperature fillings, and enzymes due to their strong tensile qualities.
The desire for CNF as a reinforcement is predicted to grow, even though the technologies often employed to synthesize CNF, such as chemical vapor deposition (CVD), are regarded as difficult and somewhat expensive.
Polymer and electrodeposition have both been intensively researched as a forerunner and as a technique.
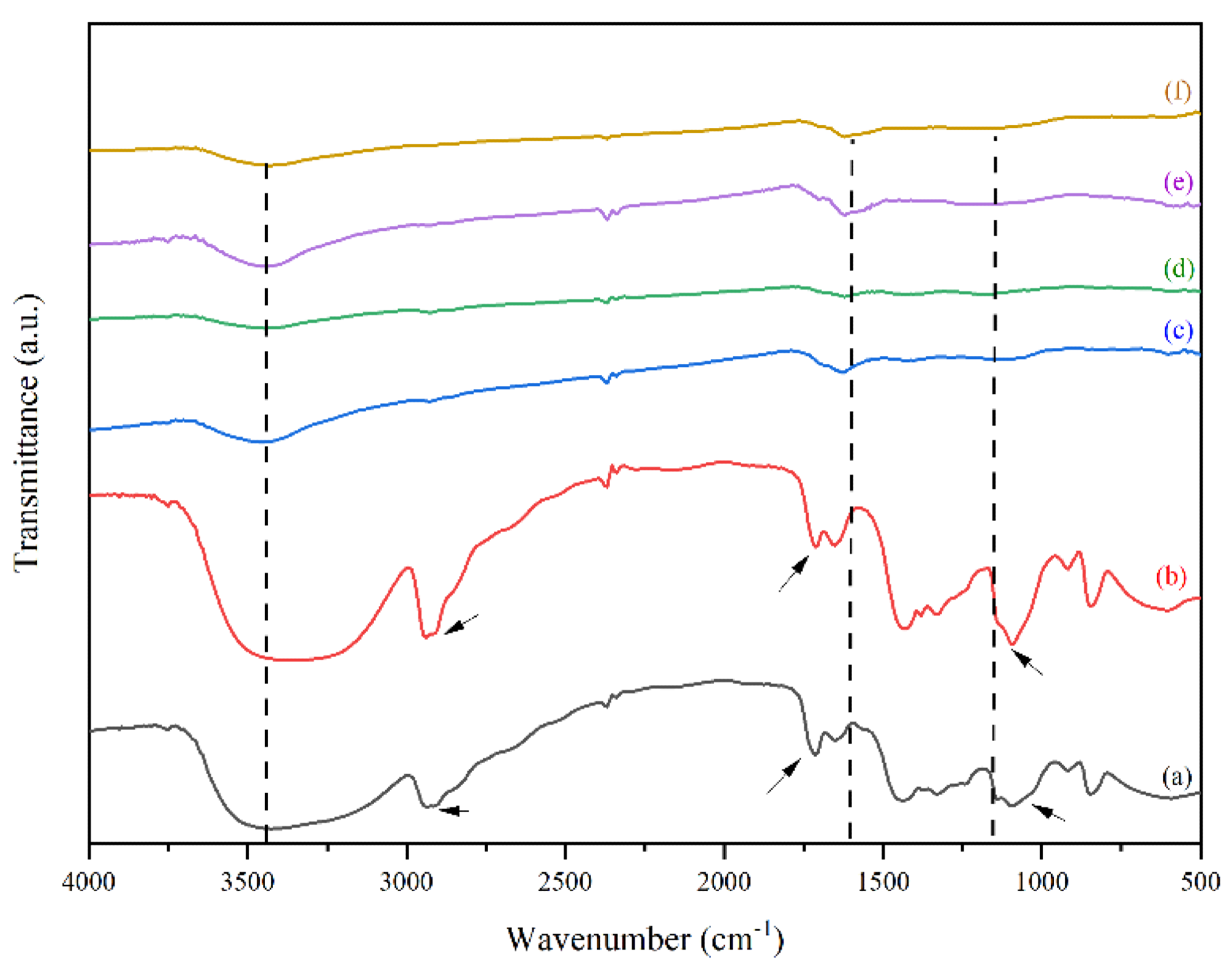
Figure 1. FTIR spectra of (a) neat PVA, (b) PVA/AgNO3 nanofiber, (c) Iodinated PVA/AgNO3 nanofiber, (d) Iodinated PVA nanofiber, (e) CNF, and (f) CNF/Ag.
© Gea S, et, al. (2022)Importance of Polymers
Polymers having long hydrocarbon groups are often utilized as intermediates in the manufacture of carbon fibers.
Numerous monomers, including poly(acrylonitrile), poly (vinyl alcohol), poly (amic acid), and phenolic resin, produced more than 50% carbon output when synthesized in cyanide acetone with acrylonitrile (HCN).
With a high carbon content of up to 54.5 percent, polyvinyl alcohol (PVA) is deemed suitable as a forerunner.
It also has a high solubility in water, is non-toxic, and works as a reducing agent. Furthermore, these properties increase the number of carbon fibers synthesized and lower the processing cost.
As a result, thermal combustion of PVA over 400 C may result in a decreased yield of CNF, necessitating the use of an alternate pre-preparation procedure to solve this problem.
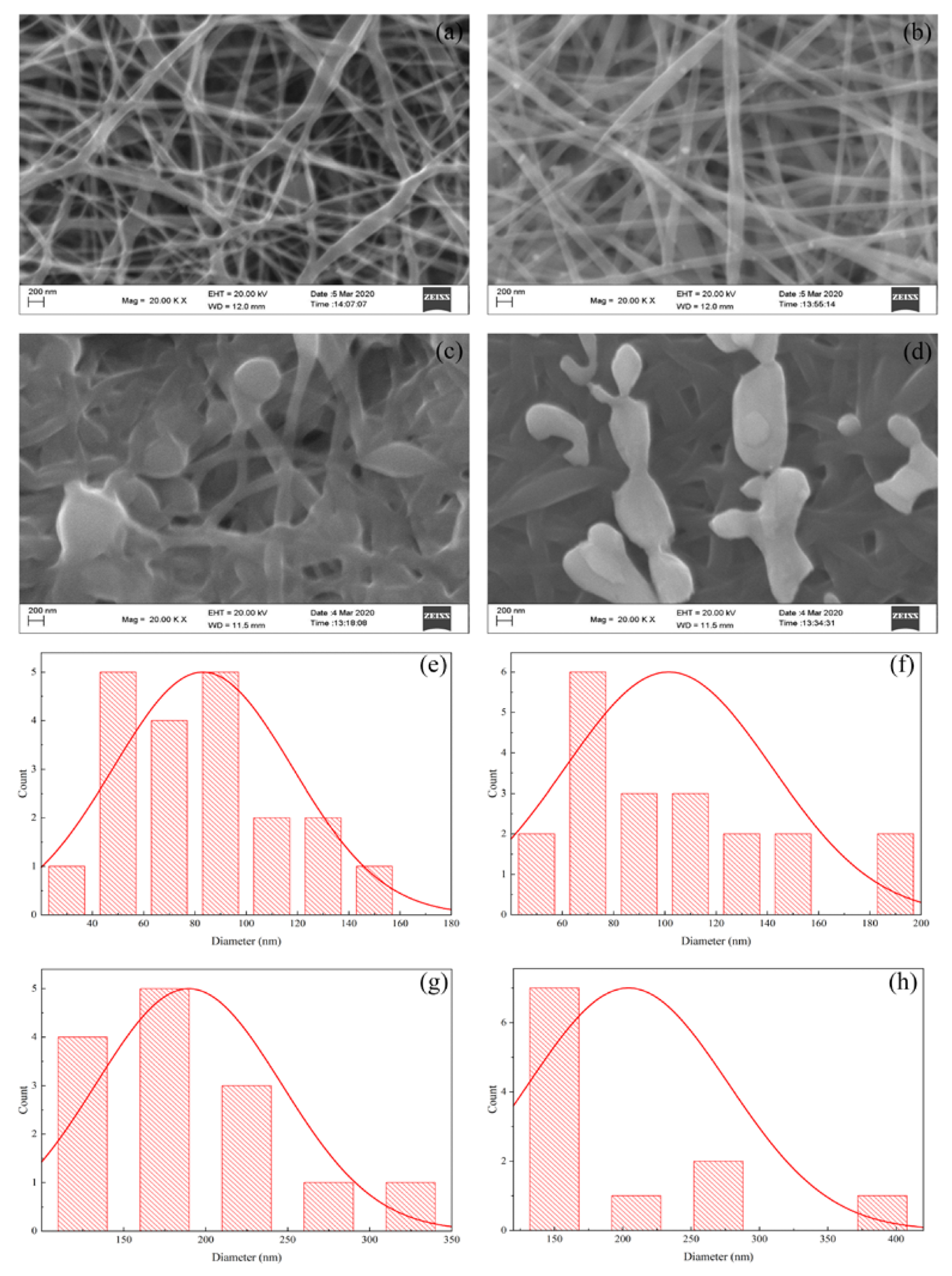
Figure 2. SEM morphological images of (a) PVA nanofiber; (b) PVA/AgNO3 Nanofiber; (c) iodinated PVA nanofiber; (d) iodinated PVA/AgNO3 nanofiber, and the diameter-size distributions of (e) PVA nanofiber; (f) PVA/AgNO3; (g) iodinated PVA nanofiber; and (h) iodinated PVA/AgNO3 nanofiber. © Gea S, et, al. (2022)
Formation of Carbon Fibres
Several attempts have been made to identify alternative pre-preparation procedures, notably those related to the functional components and the inclusion of metals via electrospinning and traditional processes.
As the synthesis of carbon fibers typically involves three processes: stabilization, carbonization, and occasionally graphitization, a sequence of pre-treatment procedures are implemented to preserve stability, resulting in an increase in CNF yield.
Metal ions have been shown to have a favourable effect on the thermal breakdown and carbonization of PVA. The metals used in electrospinning speed up the gelatinization system and improve the electrical characteristics.
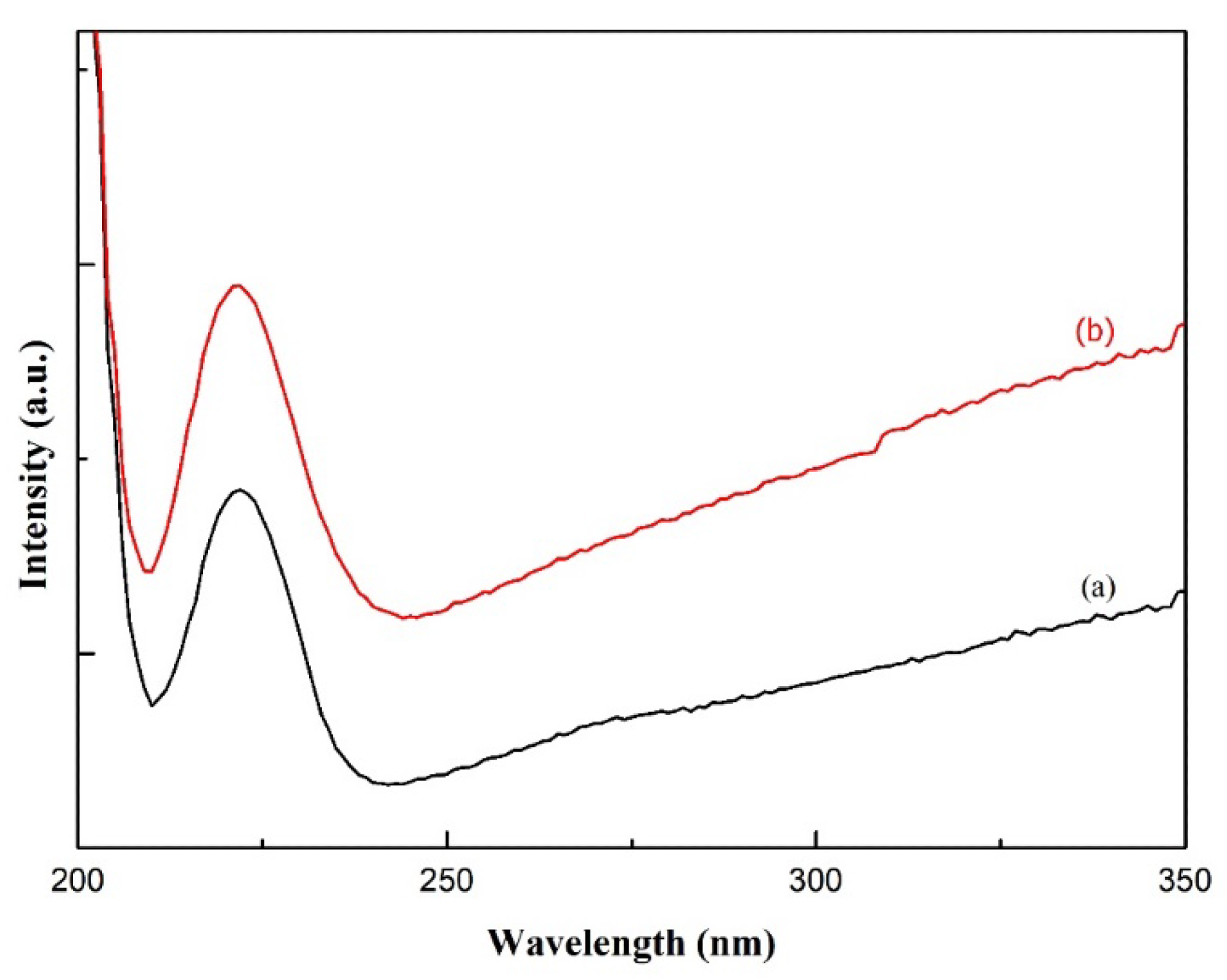
Figure 3. UV-Vis Spectra of carbon nanofibers from (a) PVA and (b) PVA/AgNO3. © Gea S, et, al. (2022)
Importance of Silver Nanoparticles
Silver nanoparticles are gaining prominence owing to their wide range of biological applications.
The antibacterial properties of silver nanoparticles have been used to suppress bacterial growth in a variety of applications, including orthopedic surgeries, mending and wound care, and health technologies.
They are the most often used sterilizing nanoparticles in commercial and medical products like textiles, tinned reusable grocery bags, refrigerator panels, and hygiene products.
These nanomaterials have been found to have enzymatic oxidative capabilities for biological agents as well as industrial compounds such as benzene. Silver nanoparticles are also commonly used as surface-enhanced Raman scattering (SERS) and metal-enhanced fluorescence (MEF) sensors (MEF).
In the most recent study, AgNO3 is used as a resource of silver nanoparticles (SNP) for the thermal cracking of PVA substrate. To develop a porous structure, iodination inside PVA and electrospinning are recommended as pre-treatment techniques.
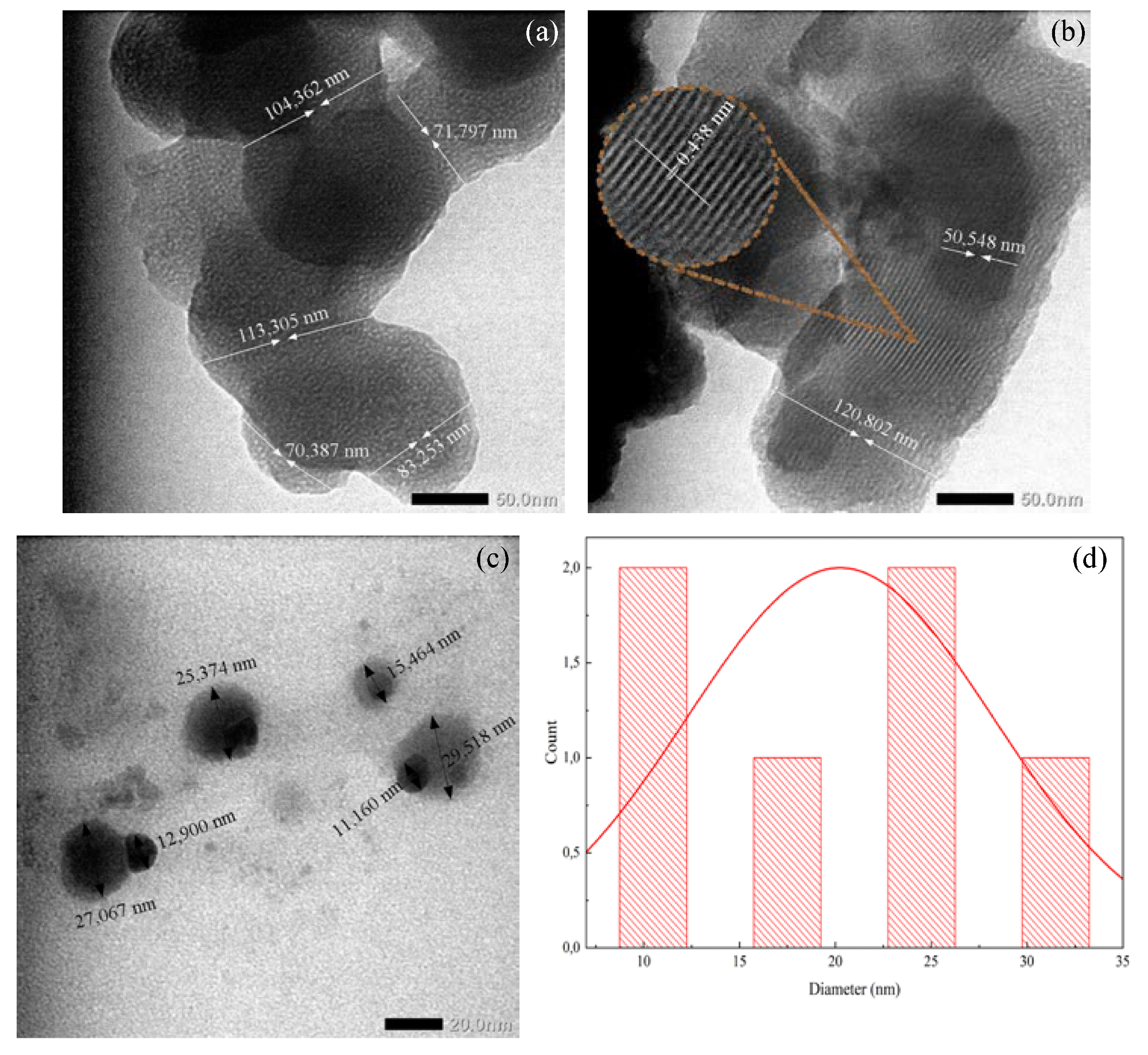
Figure 4. TEM images of (a) Carbon nanofiber from iodinated PVA nanofiber, (b) carbon nanofiber with silver nanoparticle from iodinated PVA/AgNO3, (c) silver nanoparticle, and (d) diameter particle distribution. © Gea S, et, al. (2022)
Experimental Results
When negatively charged electrons from hydrophilic groups in PVA reacted with positive ions in Ag, both physical and chemical interactions were seen.
Hydrogen bonds have been claimed to be formed between the hydroxyl groups in PVA and the polymeric units from the fluid (water), as well as the nitrate ion from AgNO3.
SEM photos demonstrated the impact of Ag and iodinated deposition on electrospun nanofibers.
The PVA polymer generated nanofibers with varying diameters ranging from 20 to 160 nm. Because the inclusion of metals improved the conductance and stickiness, the widths of the PVA/AgNO3 fibers were substantially more uniform.
The addition of AgNO3 increased the conductivity of the solution. Based on trial investigations, the quantity of AgNO3 at 0.2 percent weight fraction proved to be the best formulation where aggregation could be prevented.
Raman spectroscopy was used to determine the graphitic structure of carbon fibers. During the experiments, a one-step heating treatment was able to cut the diameter of iodinated electro-spun fibers in half.
To conclude, carbon nanofibers were effectively synthesized from PVA with the inclusion of silver nanoparticles in the form of AgNO3 via a one-step pyrolysis procedure.
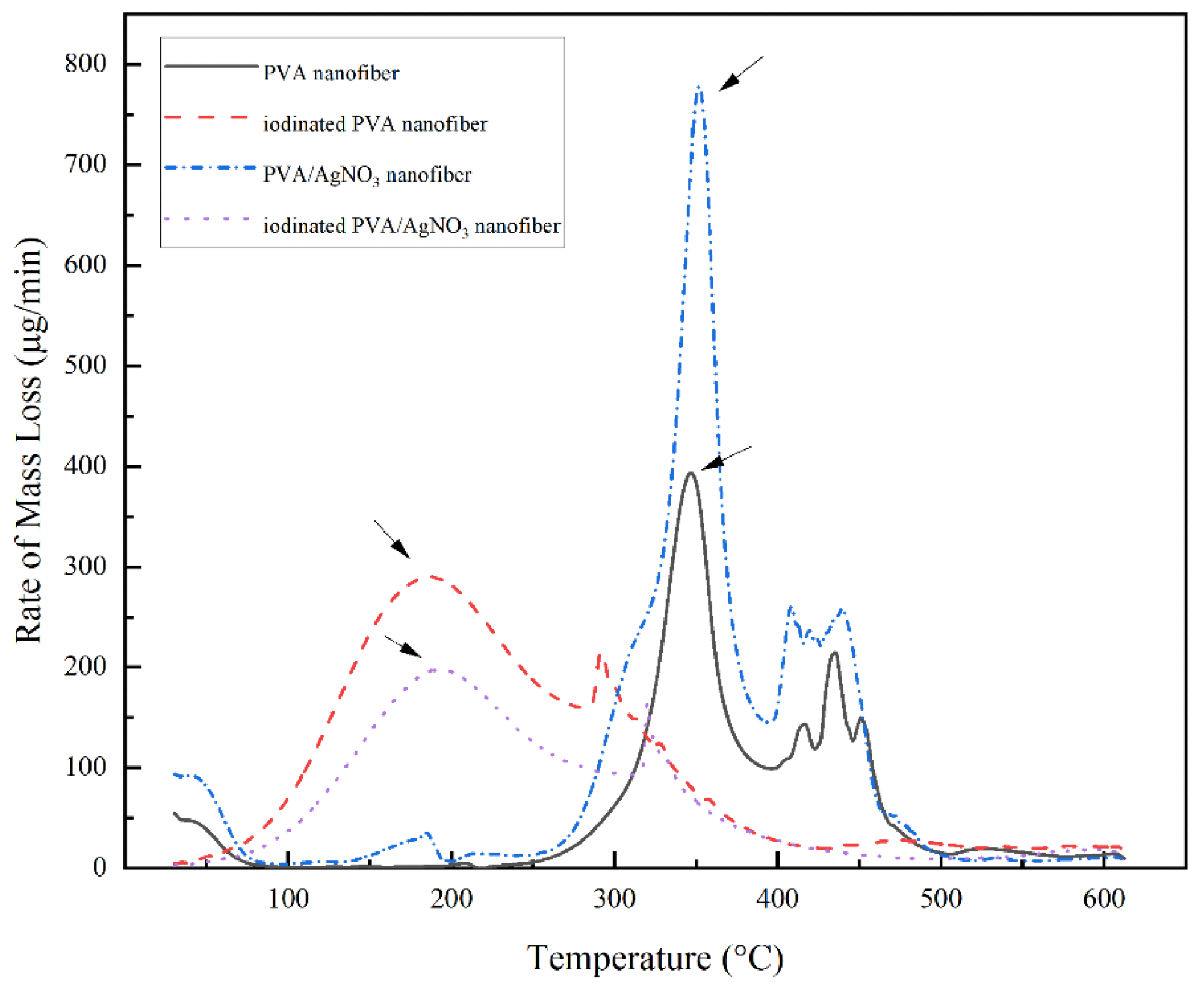
Figure 5. DTG thermogram of PVA Nanofiber, Iodinated PVA Nanofiber, PVA/AgNO3 Nanofiber, and Iodinated PVA/AgNO3 Nanofiber. © Gea S, et, al. (2022)
Continue reading: High Thermal Effects on Carbon Nanotube-Reinforced Concrete
Reference
Gea S, et, al. (2022) Carbon-Nano Fibers Yield Improvement with Iodinated Electrospun PVA/Silver Nanoparticle as Precursor via One-Step Synthesis at Low Temperature. Polymers. 14(3). 446. Available at: https://www.mdpi.com/2073-4360/14/3/446
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.