Novel research on the use of nanoflowers has been published in the journal, Pharmaceutics, for their application in cancer treatment. The research team utilized silicon-based nickel oxide nanoflowers, evaluating their potential use for therapeutic applications.
Study: Biocompatible Core–Shell-Structured Si-Based NiO Nanoflowers and Their Anticancer Activity. Image Credit: Giovanni Cancemi/shutterstock.com
Nanoflowers
This novel developed class of nanoparticles has gained attention due to beneficial characteristics such as simple preparation method, high stability and enhanced efficiency.
Interestingly, their name, ‘nanoflower’ is derivative of flowers with which they bear structural resemblances and comprise a nanosize range of 100-500 nm. They are structured to have several layers of petals to increase their surface area for versatility in applications from catalysis, biosensing and drug delivery.
While metals including gold and silver within biomaterials have been researched for their therapeutic effects, other metal oxides have also been put forth as having antimicrobial effects such as nickel oxide (NiO) and zinc oxide (ZnO).
Although ZnO nanoparticles have been widely investigated within the literature for use in cancer therapy due to their toxicity against cancer cells, they can also be harmful and illustrate general cytotoxicity in high doses.
Additionally, further research on metal oxides involving nickel has highlighted its unique optical, electronic, and physiochemical characteristics, which can be useful for a range of fields including photocatalysis, drug delivery, magnetic resonance imaging, and cancer therapy.
This ensured the researchers of the study were provided with a strong foundation for their hypothesis.
The research team aimed to investigate novel flower-like NiO nanoparticles, which demonstrated high anticancer activity while also having low cytotoxicity towards healthy cells.
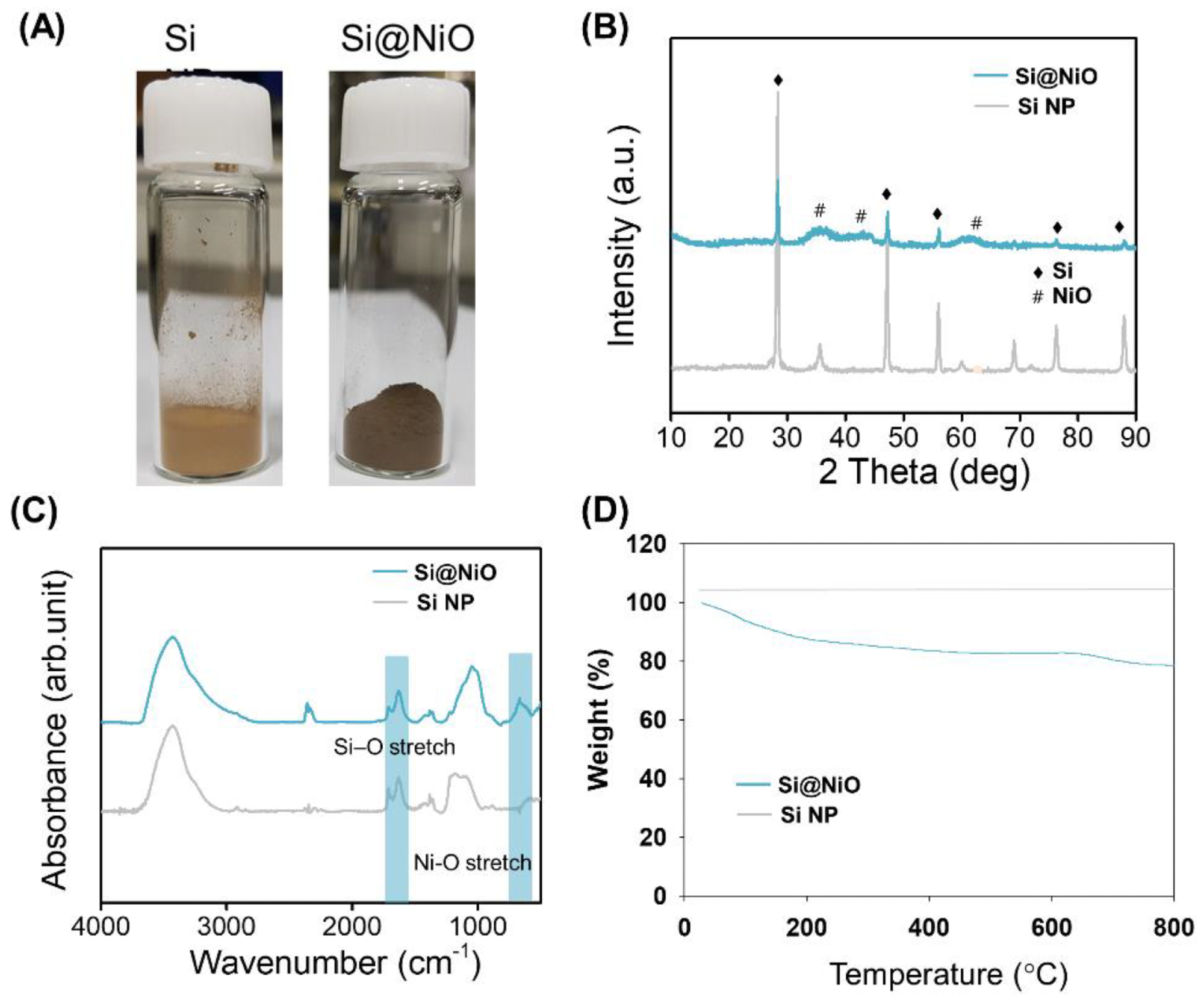
Figure 1. Characterization of Si@NiO nanoflowers. (A) Photographs of Si NP and Si@NiO. (B) PXRD patterns, (C) FT-IR spectra, and (D) TGA curves of Si NP and Si@NiO in air atmosphere. © Gwon, K., Park, J., Lee, S., Yu, J. and Lee, D., (2022)
Novel Research
The research comprised the preparation of core–shell-structured silicon-based NiO (Si@NiO) nanoflowers through using a modified chemical deposition method and then thermal reduction.
The novel Si@NiO nanoflowers were investigated with their physical and electrochemical properties being evaluated along with therapeutic potential through experiments testing the viability of the human breast cancer cell line (MCF-7).
Additionally, the cytotoxicity of these potentially revolutionary nanoparticles was also evaluated using mouse embryonic fibroblasts (MEFs). The results of the experiment illustrated high anticancer activity within the breast cancer cell line as well as low cytotoxicity within the mouse embryonic fibroblasts.
The selective nature of these novel Si@NiO nanoflowers may have been attributed to reactive oxygen species due to the redox reaction of ascorbic acid with the metal NiO ions on the surface of the core–shell nanoflowers.
This may have led to more cytotoxicity in the cancerous cells compared to the healthy cells.
The increase in reactive oxygen species to a level that results in toxicity in cancer cells may be an effective and efficient approach for cancer treatment. Cancer cells are eliminated via active oxygen-mediated apoptosis or through inhibiting cancer cell resistance to chemotherapy.
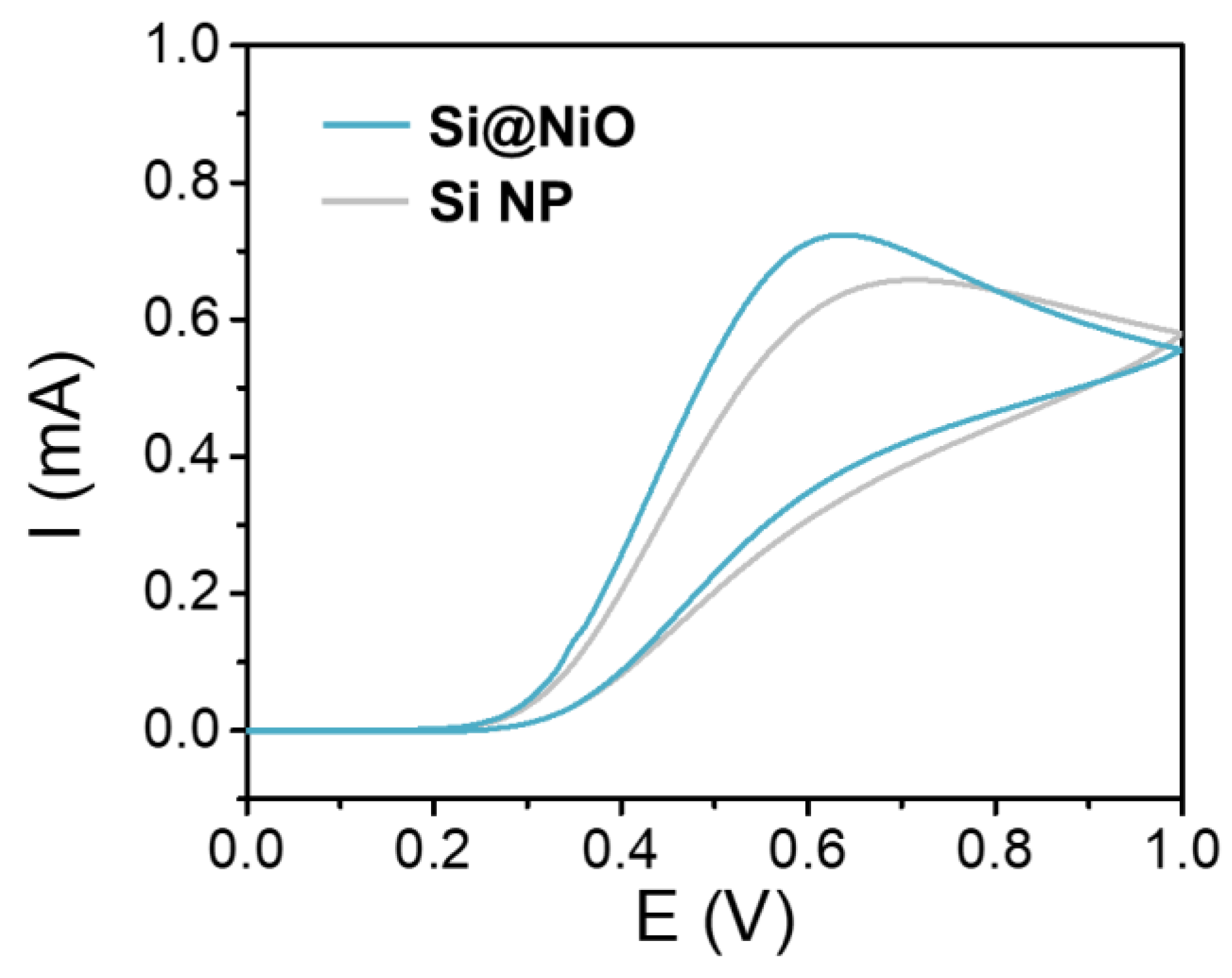
Figure 2. Electrochemical characterization. CV curves of Si NP and Si@NiO acquired in PBS containing 0.1 M AA; scan rate: 100 mVs−1. © Gwon, K., Park, J., Lee, S., Yu, J. and Lee, D., (2022)
Implications for Cancer Therapeutics
The two most important requirements the researchers were aiming to achieve for enhanced anticancer activity included the novel nanoflowers having (i) high efficiency as well as (ii) selective toxicity.
These factors are significant for cancer research, as targeted cancer therapy that aims to save and preserve healthy cells and tissue would ensure patients are provided with a better quality of treatment as well as life.
This would prevent the needless destruction of healthy organs which can become dysfunctional after chemotherapy treatment, even resulting in the requirement of organ transplants.
The use of chemotherapy or anticancer drugs can cause adverse effects to the lungs, heart, metabolism, kidneys, nervous system and bone marrow, illustrated by an anticancer antibiotic which can result in lung toxicity in patients with an incidence rate between 2% and 46%.
The success of being highly effective as well as selective for the treatment of cancerous tissue would alleviate the burdens and trauma associated with cancer therapeutics; this is due to patients not always living the most fulfilled life after cancer treatment and requiring long recovery times due to high systemic toxicity.
Advancements in nanotechnology through the use of nanoflowers and surface functionalization of various metal oxides such as NiO, allows further research into cancer therapeutics which aim to prioritize cancer patients and provide them with precision medicine. This type of research is crucial for advancing the field of medicine and pharmaceuticals as these industries hold significance to the quality of care provided to vulnerable patients.
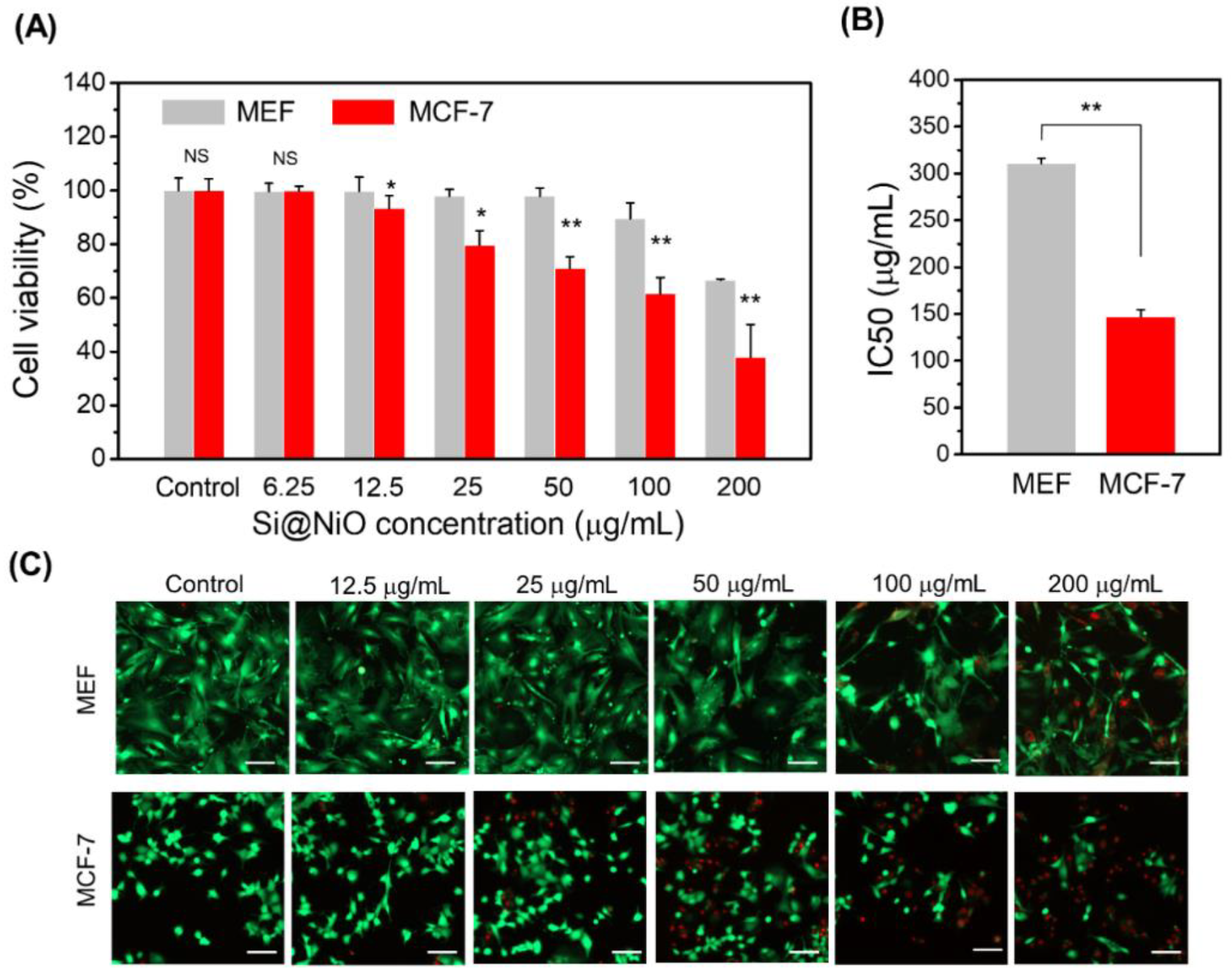
Figure 3. Cytotoxicity and anticancer activity of Si@NiO nanoflowers. (A) Cytotoxicity of Si@NiO toward the MEF and MCF-7 cells after 24 h of incubation. (B) IC50 values of Si@NiO in the MEF and MCF-7 cells. (C) Live/dead staining images of MEFs and MCF-7 after incubation with various concentrations of Si@NiO for 24 h. Positive control: cells cultured in the absence of Si@NiO. NS: not significant, * p < 0.05 and ** p < 0.01. Scale bar: 100 μm. © Gwon, K., Park, J., Lee, S., Yu, J. and Lee, D., (2022)
Reference
Gwon, K., Park, J., Lee, S., Yu, J. and Lee, D., (2022). Biocompatible Core–Shell-Structured Si-Based NiO Nanoflowers and Their Anticancer Activity. Pharmaceutics, 14(2), p.268. Available at: https://www.mdpi.com/1999-4923/14/2/268/htm
Further Reading
Clinical Gate. (2022). Organ Toxicity of Cancer Chemotherapy. [online] Available at: https://clinicalgate.com/organ-toxicity-of-cancer-chemotherapy/
Shende, P., Kasture, P. and Gaud, R., (2018) Nanoflowers: the future trend of nanotechnology for multi-applications. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup1), pp.413-422. Available at: 10.1080/21691401.2018.1428812
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.