Researchers from Germany have presented a thorough evaluation of plasmonic materials utilized as solar fuels and for hydrogen production as well as reducing levels of carbon dioxide, publishing their research in the journal ACS Energy Letters.

Study: Hybrid Plasmonic Nanomaterials for Hydrogen Generation and Carbon Dioxide Reduction. Image Credit: HaHanna/Shutterstock.com
Increment in Global Warming
The tremendous and continuous growth in energy use over the last two centuries has resulted in a worldwide resource and environmental catastrophe.
Almost 75 percent of human-caused carbon dioxide (CO2) production during the last few years has been attributed to traditional energy sources.
A shift towards alternative energy sources is significant, with many industries investing in mitigation strategies.
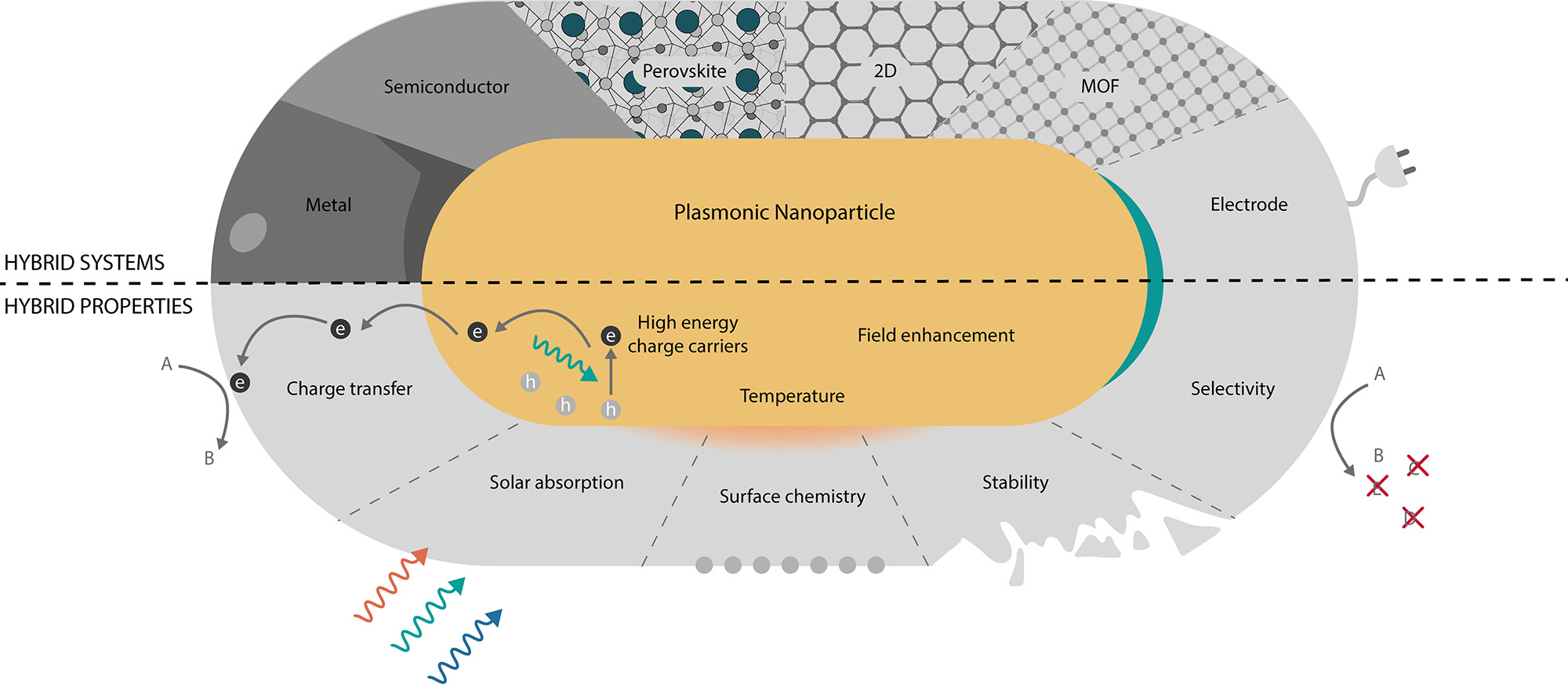
Figure 1. Hybrid plasmonic photocatalysts. (Top) Overview of the hybrid plasmonic systems that are the topic of this Review. From left to right: metal, semiconductor, perovskite, 2D, MOF, and electrochemistry. (Bottom) Desirable properties of an ideal photocatalyst. The plasmonic component can harvest light and transfer the energy through charge carriers, heat, and electromagnetic fields. Non-plasmonic materials can add or enhance catalytic properties. The hybrid system can show enhanced stability, selectivity, or activity through various mechanisms, e.g., effective charge transfer, surface chemistry, or enhanced absorption.© Ezendam, S. et al., (2022)
What are Solar Fuels?
Nature has exploited the sun as its major energy source for thousands of years through photosynthesis, in which light is utilized to divide moisture energy-rich chemical molecules from CO2.
Taking cues from this, artificial photosynthesis has emerged as one of the most prominent study areas in the last decades.
It primarily comprises of two types of operations: water breaking into hydrogen (H2) and oxygen (O2) or H2 synthesis from H2-containing molecules, and reduction of carbon into fuel sources such as carbon monoxide, hydrocarbons, and displaces oxygen. These are commonly referred to as solar fuels.
Importance of Plasmonic Nanoparticles
The initial stage in photocatalytic water splitting is to capture and condense sun energy in cellular areas.
Because of their excellent absorbance and capacity to manipulate light and heat at the nanoscale, plasmonic nanoparticles (PNPs) are intriguing substances for these activities.
As a result, including a plasmonic substance into a multifactorial nanoparticle can significantly improve its photo electrocatalytic performance when compared to the individual components.
Recently, a growing percentage of these hybrid plasmon nanoparticles have been studied for biochemical processes, spawning the area of plasmon-assisted catalysis.
Plasmon Metal Hybrids Utilization
Plasmonic substances can efficiently utilize late metallic elements for more effective photocatalytic hydrogen production.
One of the most difficult issues for these so-called photonic alloyed devices is designing the nanomaterials to leverage both photovoltaic performance and enzymatic capabilities.
PNP's required to create energizing transportation when lighted have been used in multielectron methods such as carbon dioxide reduction in recent years.
However, there appears to be no proof of plasmon hybrids bimetal photosensitizers being used in this process.
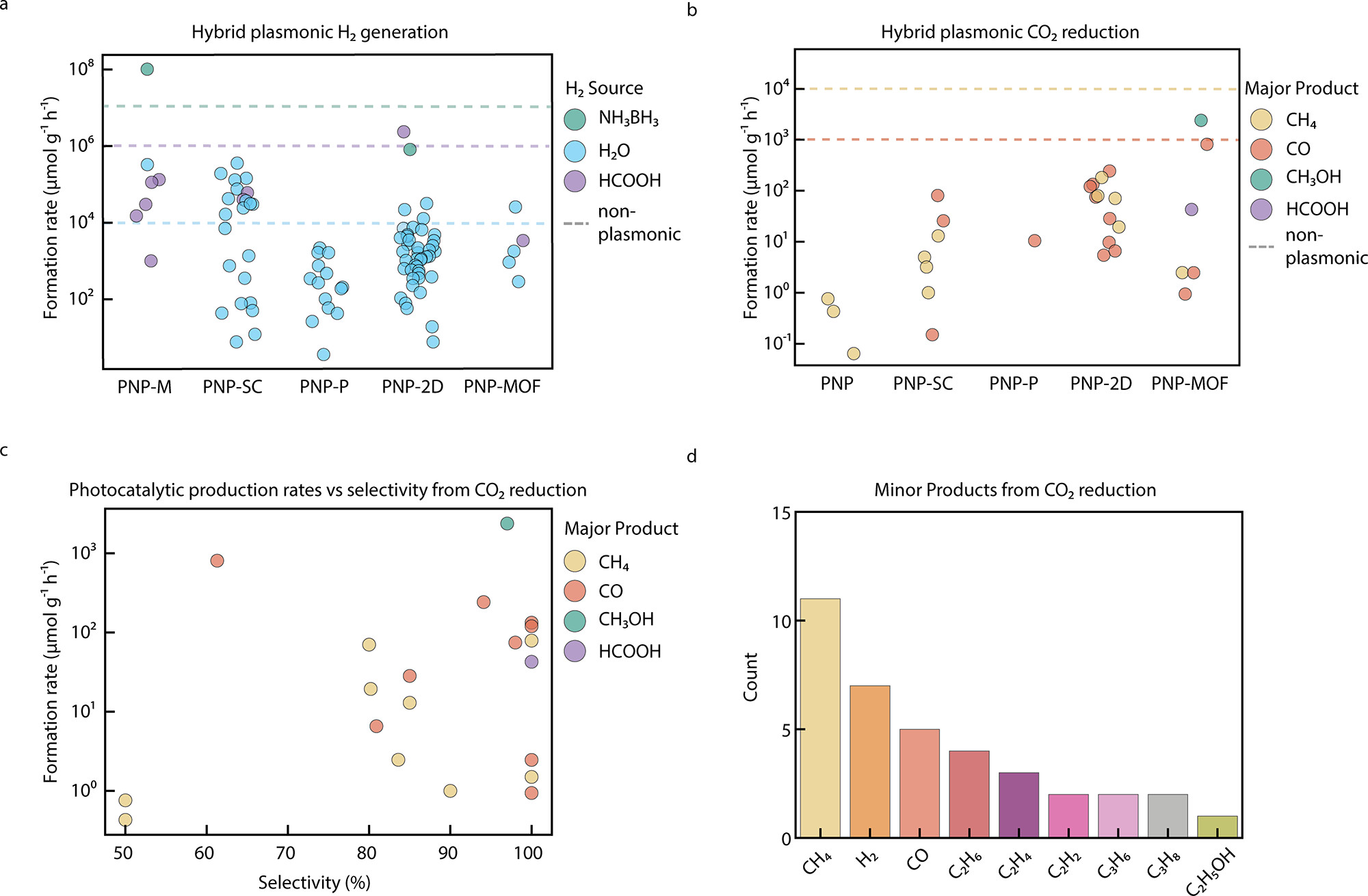
Figure 2. Hybrid plasmonic nanomaterials for the generation of solar fuels. Formation rates for (a) H2 generation and (b) CO2 reduction for the different hybrid photocatalysts reviewed here. The dashed line illustrates the order of magnitude achieved with prominent examples of non-plasmonic catalysts. (c) Formation rate vs selectivity and (d) histogram of the number of publications vs the minor products reported. © Ezendam, S. et al., (2022)
Effectiveness of Plasmon Metal Semiconductors
The transfer of electrons from a plasmonically stimulated metal particle to a semiconductor has aided in the photocatalysts generation of solar fuels.
The quest for an optimal composite photosensitizer has resulted in the coupling of PNPs with a variety of semiconductors, including TiO2, CdS, and Cu2O.
Because of its distinctive photocatalytic and photovoltaic properties, TiO2 was used as the semiconductor’s ingredient in more than 60% of the combinations for plasmonic aided photo electrocatalytic H2 generation.
The formation of a nanocomposite between metals and semiconductors is a viable technique for suppressing the HER.
The behavior and sensitivity of the response can both be optimized to favor a required product of the reaction.
Researchers performed a thorough investigation in which they discovered that the sensitivity and specificity of TiO2 with (101) angle increased with the photodegradation of AuPd nanoalloys of diverse ratio components.
The AuPd compositions with the stoichiometry of 6:1 produced the highest specificity toward molecules of 85 percent.
Usage of Plasmon Metal-Perovskite Material
The research of alternative geometrical and mechanical configurations has come from the complex formation of combining the light-harvesting characteristics of PNPs with the optoelectrical and chemical characteristics of perovskites.
Expanding optical absorption into the visual spectrum with plasmon composites is a potential technique for enhancing the efficiency of solar-to-fuel transformation.
Combining a large bang gap perovskite with a plasmon material enables photocatalytic H2O oxidation using just visible light.
As most plasmon technologies are limited to metallic nanoparticles, large-scale manufacturing applications are too costly. As a result, there has been a hunt for less expensive plasmonic replacements.
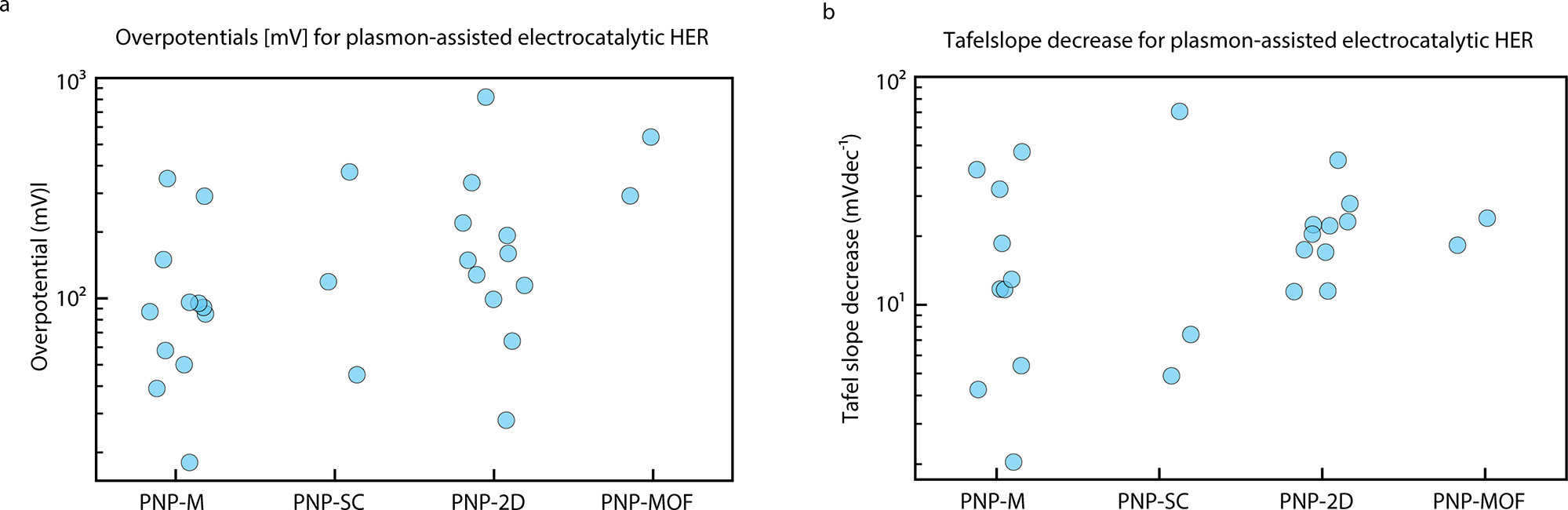
Figure 3. (a) Overpotential for plasmon-assisted electrocatalytic HER for all hybrid systems reviewed here. (b) Tafel slope decrease upon illumination of hybrid plasmonic electrocatalysts. © Ezendam, S. et al., (2022)
The use of a plasmon substance in conjunction with a perovskite for photocatalytic activity is useful not only for H2 generation but for many other photocatalytic activities such as dyes or isopropyl alcohol decomposition or carbon dioxide reduction.
Additionally, greater visual transmittance and effective charge segregation are linked to increased photocatalysts reduction activity in plasmon photocatalysis using perovskite oxides.
In short, the combinations of plasmon nanomaterials with other materials have been successful in hydrogen generation and carbon dioxide reduction.
Continue reading: Nanoscale Characterization of Polymer Thin Films with AFM.
Reference
Ezendam, S. et al., (2022) Hybrid Plasmonic Nanomaterials for Hydrogen Generation and Carbon Dioxide Reduction. ACS Energy Letters, Volume 7, pp. 778-815. Available at: https://pubs.acs.org/doi/10.1021/acsenergylett.1c02241
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.