A group of researchers recently published a paper in the journal Materials that demonstrated the viability of using vancomycin-functionalized gold nanoparticles (V-GNPs) against pathogenic bacterial strains.
Study: Enhancement of Vancomycin Potential against Pathogenic Bacterial Strains via Gold Nano-Formulations: A Nano-Antibiotic Approach. Image Credit: peterschreiber.media/Shutterstock.com
Background
Antibiotic resistance among pathogenic bacteria is rising at a remarkable rate, which poses a significant risk to human health. For instance, vancomycin, a glycopeptide antibiotic, is typically used for treating bacterial infections such as methicillin-resistant Staphylococcus aureus (MRSA). However, the excessive use of vancomycin has led to the emergence of vancomycin-resistant bacterial strains.
Innovative strategies such as the use of nanoparticles (NPs) hold the potential to combat antibiotic-resistant bacterial infections.
The characteristics of NPs, such as surface chemistry, size, and shape, can be manipulated easily, which makes them suitable for fighting bacterial infections.
Metallic NPs, such as GNPs, are specifically being evaluated in different studies for their potential in treating bacterial infections.
Several studies have shown that the antibacterial activities of antibiotics can be enhanced by conjugating GNPs with antibiotics.
In this study, researchers investigated the ability of GNPs to enhance the antibacterial efficacy of the antibiotic vancomycin against different bacterial strains.
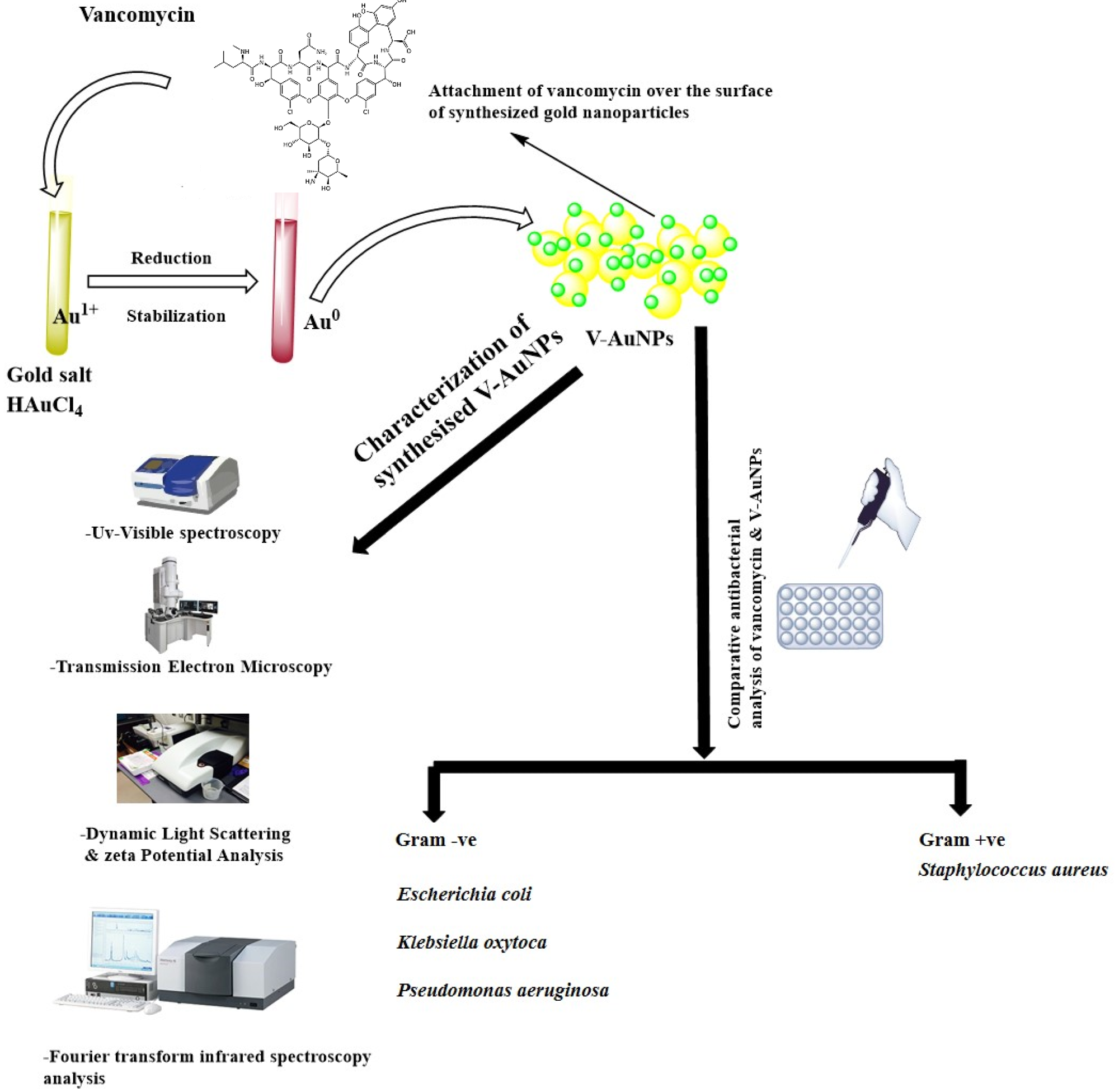
Figure 1. Schematic representation of vancomycin-mediated synthesis of gold nanoparticles (V-GNPs), their characterization, and antibacterial testing. © Hagbani, T.A., Yadav, H., Moin, A. et al. (2022)
The Study
Gold (III) chloride trihydrate (HAuCl4·3H2O), phosphate buffer, and vancomycin were used for preparing V-GNPs in the study.
In the preparation, 1, 0.75, 0.50, or 0.25 mg/mL of vancomycin was mixed with a 3 mL reaction mixture containing 50 mM phosphate buffer with a pH value of 7.4 and 1 mM HAuCl4·3H2O, and the resultant mixture was incubated at 60, 50, 40, and 30 oC, respectively, for 48 h.
The synthesized V-GNPs were obtained by centrifugation of the reaction mixture for 30 min at 30,000× g and the collected NPs were treated with 50% v/v ethanol in order to eliminate unattached materials.
The Gram-positive bacterial strain of Staphylococcus aureus and the Gram-negative strains of Pseudomonas aeruginosa, Klebsiella oxytoca, and Escherichia coli were used in the study to evaluate the antibacterial efficacy of V-GNPs.
Each bacterial strain was maintained and cultivated in Mueller Hinton (MH) agar media at 37 oC.
A Shimadzu UV-1601 spectrophotometer was employed to obtain the ultraviolet ultraviolet-visible (UV-vis) spectra of the prepared V-GNPs in the range of 200-800 nm at 1 nm resolution.
The average particle size of the synthesized V-GNPs was determined using a dynamic light scattering particle (DLS) size analyzer, while the zeta-potential of the synthesized NPs was measured using a Malvern Zetasizer Nano-ZS.
A transmission electron microscopy (TEM) operating at an accelerating voltage of 80 kV was used to assess the homogeneity of the prepared V-GNPs, while Fourier transform infrared (FTIR) spectroscopy was performed to evaluate the conformational changes after vancomycin was loaded on the surface of GNPs.
The FTIR spectra were acquired using the potassium bromide (KBr) pellet method with a Shimadzu FTIR-8201 spectrometer within the range of 4000–400 cm-1 at a resolution of 4 cm-1.
The agar well diffusion methodology was utilized to evaluate the efficacy of prepared V-GNPs and pure vancomycin.
The in vitro antibacterial activity of pure vancomycin and V-GNPs was assessed using the minimal inhibitory concentration (MIC) method. The loading efficiency of V-GNPs was also calculated. All findings in the study were analyzed using GraphPad Prism through a one-way analysis of variance (ANOVA).
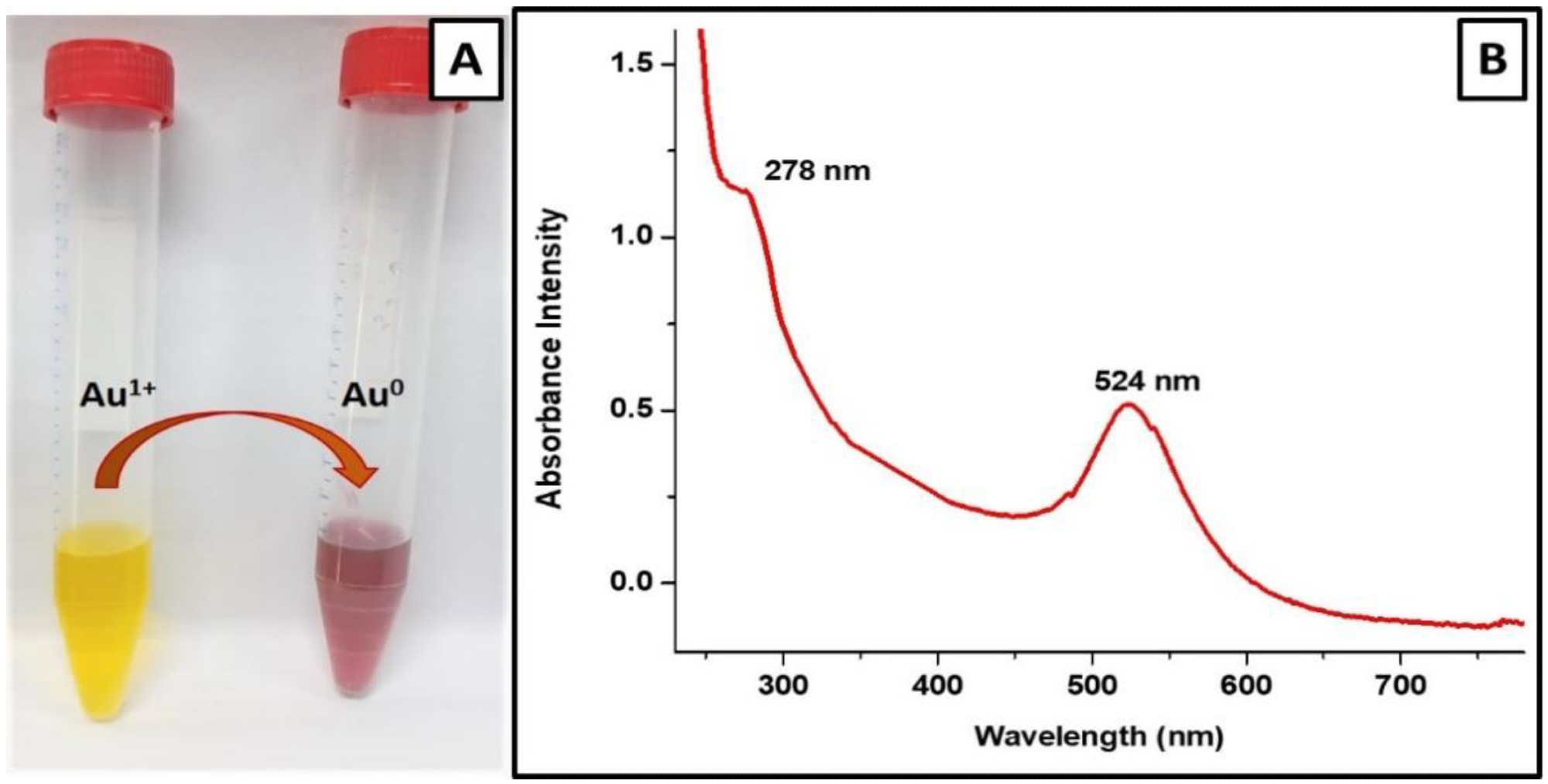
Figure 2. Characterization of V-GNPs: (A) color change from light yellow to ruby red resulted from SPR; (B) UV–Visible spectra (SPR band at 524 nm). © Hagbani, T.A., Yadav, H., Moin, A. et al. (2022)
Observations
GNPs with good physicochemical properties were synthesized at a pH of 7.4, a temperature of 40 oC, and a vancomycin concentration of 250 µg/mL.
The color of the gold salt solution changed from pale yellow to ruby red following the addition of 250 µg/mL vancomycin antibiotic, indicating the formation of V-GNPs.
The UV-Vis spectra of the prepared V-GNPs revealed the presence of a surface plasmon resonance peak at 524 nm.
The TEM micrographs showed that the synthesized V-GNPs were monodispersed, homogenous, and spherical in shape with an average size of 24 nm. No agglomeration was observed in the TEM micrographs, indicating the suitability of vancomycin as a stabilizing agent.
The hydrodynamic diameter of the V-GNPs was estimated as 77 nm by the DLS.
The zeta potential of the prepared V-GNPs was -18mV, indicating good stability of GNPs. Observations from the FTIR spectroscopy indicated that the vancomycin was efficiently loaded on the GNP surfaces, and the loading efficiency was 86.2%.
Both V-GNPs and pure vancomycin significantly inhibited bacterial growth. However, V-GNPs demonstrated more effective antibacterial activity compared to pure vancomycin even at a lower concentration of vancomycin.
The in vitro antibacterial studies demonstrated that the antibacterial activities of the synthesized V-GNPs were 1.6-, 1.8-, 1.6-, and 1,4-fold higher than pure vancomycin against Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella oxytoca, and Escherichia coli, respectively.
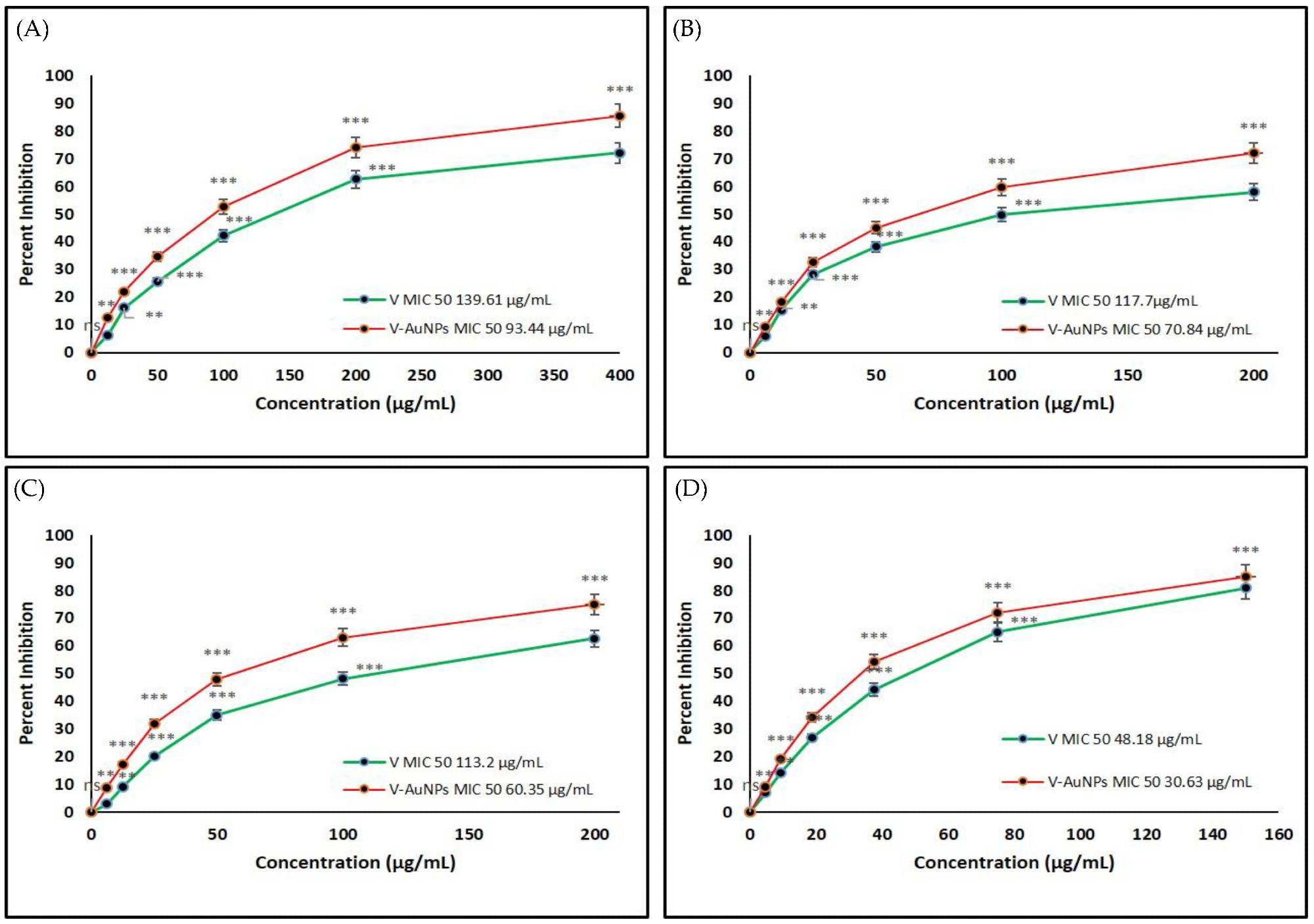
Figure 3. Determination of the minimum inhibitory concentration (MIC) of vancomycin (V) and V-GNPs against (A) Escherichia coli; (B) Klebsiella oxytoca; (C) Pseudomonas aeruginosa; (D) Staphylococcus aureus. The experiment was repeated in triplicate, and the data shown are the means ± standard errors. Significantly different from control at ** p < 0.01, *** p < 0.001; non-significant from the control at ns p > 0.05. © Hagbani, T.A., Yadav, H., Moin, A. et al. (2022)
Significance of the Study
Taken together, the findings of the study demonstrated that V-GNPs represent a more suitable alternative compared to pure vancomycin against pathogenic bacterial strains.
However, in vivo investigations must be performed to assess the toxicity of V-GNPs before the synthesized vancomycin nanoformulations can be used to treat infections. Nevertheless, this study can act as a starting point for developing better antibacterial nanoformulations.
Reference
Hagbani, T.A., Yadav, H., Moin, A. et al. (2022) Enhancement of Vancomycin Potential against Pathogenic Bacterial Strains via Gold Nano-Formulations: A Nano-Antibiotic Approach. Materials, 15, 1108. Available at: https://www.mdpi.com/1996-1944/15/3/1108/htm
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.