Currently, the fabrication and development of particles with nanopatterned surfaces have gained considerable attention due to their application in the self-assembly of multifaceted hierarchical structures.
Particles with nanopatterned surfaces are referred to as particles with topographically or chemically distinct surface patches. The patches provide localized interaction sites that promote the self-assembly of the structures.
Effective methods were already developed for synthesizing sub-micrometer- and micrometer-sized patchy colloids. Thus, the current efforts are mainly directed toward the generation of patchy NCs, with sizes in the order of tens of nanometers.
Recently, patchy lanthanide fluoride (LnF3) NCs were synthesized successfully, with the patches displaying various electric charges due to the selective functionalization of different NC patches with cationic and anionic ligands.
However, the patchy distribution of ligands, as predicted molecular dynamics (MD) simulations, cannot be detected by the previously used experimental techniques such as infrared (IR) and hydrogen-1 nuclear magnetic resonance (1H-NMR) spectroscopy as these techniques lacked spatial resolution.
The location of the patches on the NC surface and their nanometric size, which is less than 10 nm, are the major challenges in the detailed characterization of these patches.
Developing methods that can provide a reliable characterization of the NC patches are necessary to ensure rational and reproducible synthesis of patchy NCs.
In this study, researchers identified the most suitable method to obtain a tunable range of LnF3 NC sizes between 5 and 15 nm by evaluating different synthesis strategies and performed a real-space characterization of the synthesized LnF3 patchy NCs through different microscopy techniques.
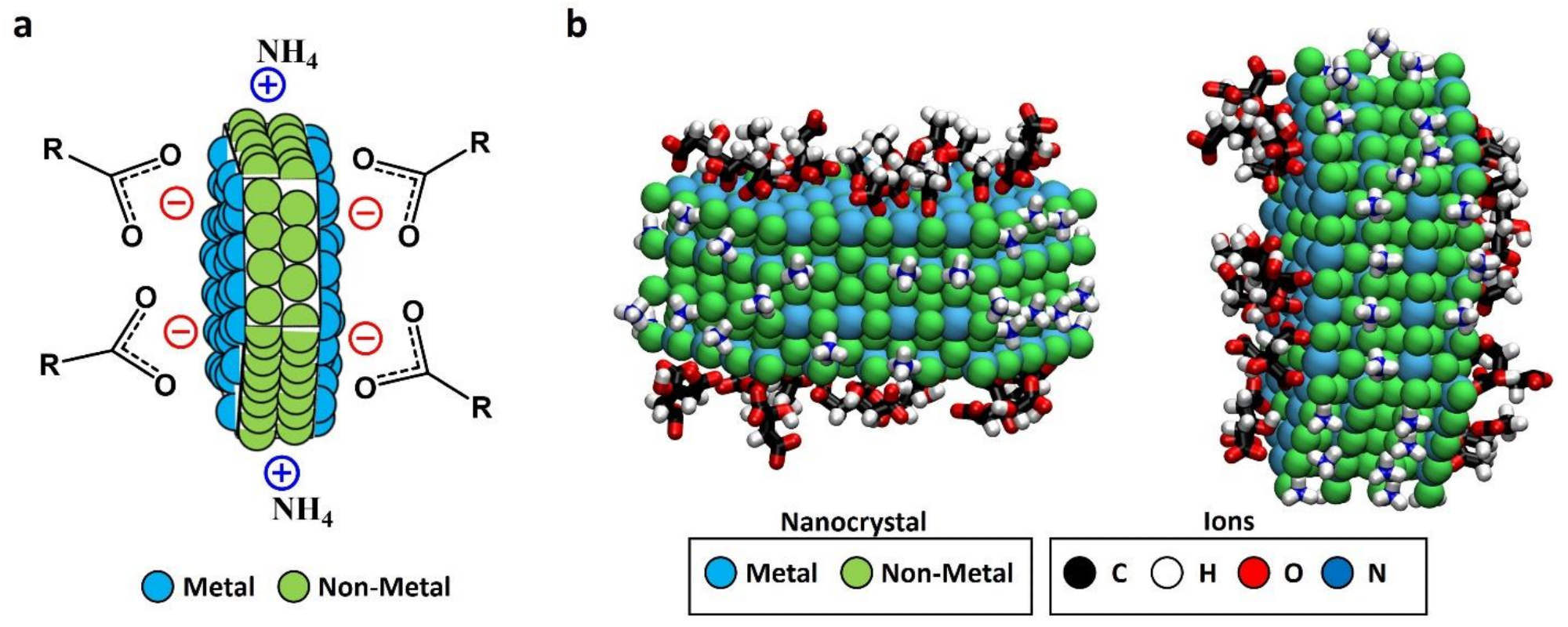
Figure 1. (a) Schematic representation of faceted-charge patchy NC with the local charges in the exposed patches due to the ionic stabilizers; (b) snapshot (two different views) generated from the results of our previous MD simulations of a LaF3 NC in solution (NC atoms are shown as van der Waals spheres, and adsorbed ions are shown in licorice representation; water and non-adsorbed ions are not shown for simplicity). The images show adsorbed acetate and citrate anions (found only at the hexagonal face) and adsorbed ammonium cations (found only at the rectangular face), thereby predicting the formation of charged patches due to ligand adsorption. These adsorptions gave a charge density of approximately −3.4 e/nm2 for hexagonal facets and +3.4 e/nm2 for the rectangular ones, obtaining a global charge density of −0.47 e/nm2 considering the NC. Images in (b) were generated with Visual Molecular Dynamics software (VMD) version 1.9.3 (Urbana—Champaign, IL, USA). © Martínez-Esaín, J., Pérez-Rodríguez, A., Faraudo, J. et al. (2022)
The Study
Cerium (III) acetate hydrate, lanthanum (III) acetate hydrate, ammonium fluoride, tetramethylammonium hydroxide 25 wt.% in water, citric acid, and acetone were used as starting materials.
Cerium fluoride (CeF3) and lanthanum fluoride (LaF3) NCs were synthesized using microwave reaction method, hydrothermal method, and coprecipitation method followed by hydrothermal post-treatment.
A Phillips XPert diffractometer with a copper (Cu) tube and two circle diffractometers was employed to record the X-ray diffraction (XRD) patterns of the synthesized samples. A 120 kV JEOL 1210 transmission electron microscope (TEM) and a 200 kV JEOL 2011 TEM were utilized to obtain the TEM and high-resolution TEM (HRTEM) images of the NCs.
A Milestone model FlexiWAVE microwave oven was used to heat the NCs at 200 oC. The QUANTA FEI 200 FEG-ESEM was used to perform scanning electron microscopy (SEM) analyses of the samples, while atomic force microscopy (AFM) measurements were performed using the commercial control and head unit from Nanotec Electrónica under ambient conditions and at room temperature.
Kelvin probe force microscopy (KPFM) was utilized in the frequency modulation mode (FM-KPFM), where the tip was excited by 0.5 AC voltage at 0.7 kHz frequency and a feedback loop was used to adjust the DC bias required to reverse the frequency shift (∆f) at fAC.
Multiple surface locations were measured using the same tip for every NC containing sample for at least a single set of experiments.
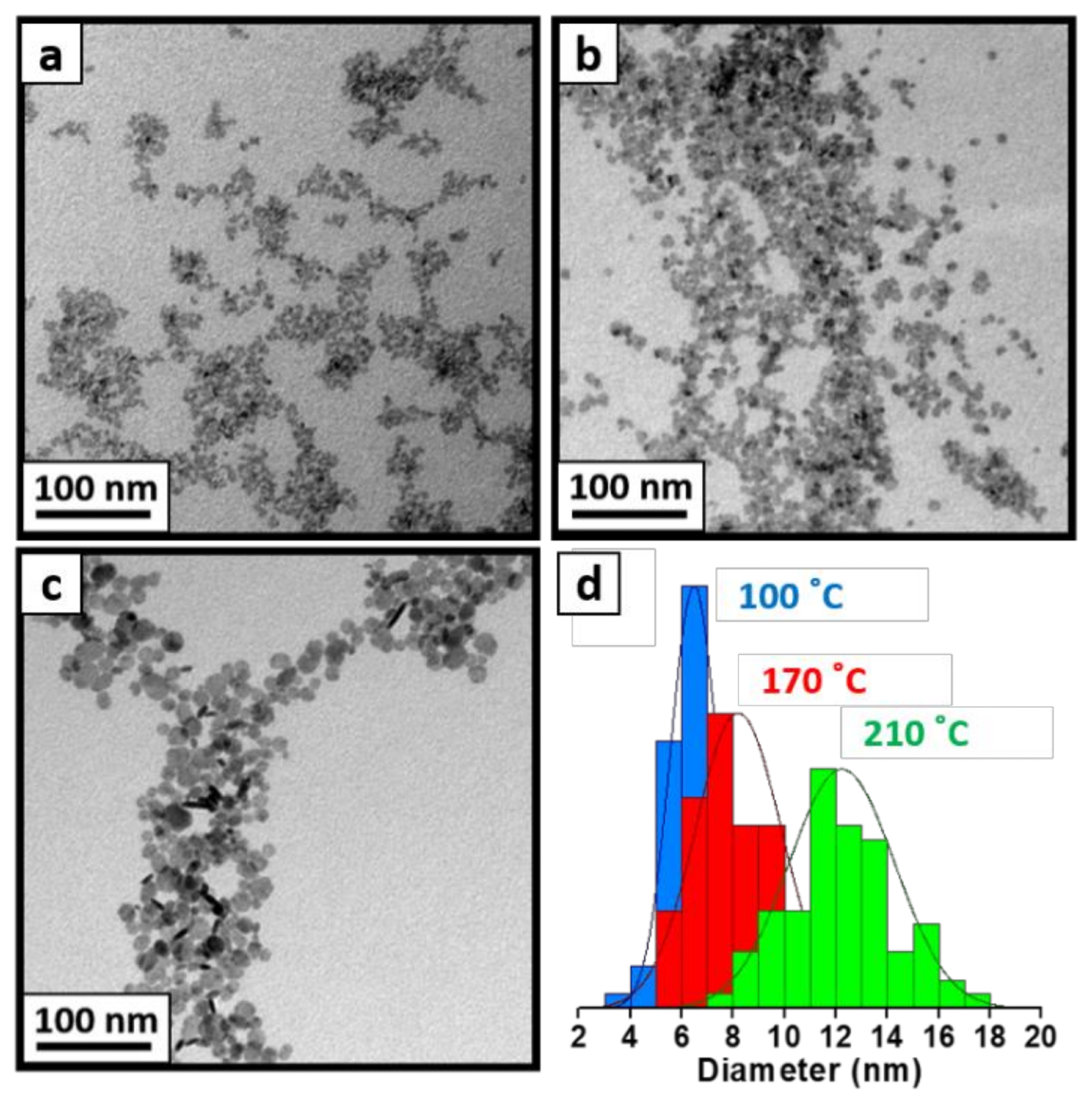
Figure 2. TEM images of as-synthesized LaF3 patchy NCs via coprecipitation method at 100 °C (a) and after a hydrothermal post-treatment at 170 °C (b) and 210 °C (c). TEM histograms (d) of three images are also shown to corroborate their increasing size: as-synthesized LaF3 NCs (blue), LaF3 after hydrothermal post-treatment at 170 °C (red), and LaF3 after a hydrothermal post-treatment at 210 °C for two additional hours (green). © Martínez-Esaín, J., Pérez-Rodríguez, A., Faraudo, J. et al. (2022)
Observations
Faceted-charge patchy NCs with sizes in the tunable range were synthesized successfully.
The final sizes of LaF3 NCs synthesized by the coprecipitation method followed by a hydrothermal treatment were 8 nm and 12 nm at 170 oC and 210 oC temperatures, respectively, while the final sizes of LaF3 NCs synthesized by the microwave reaction and direct hydrothermal method at similar temperatures were in the range of 13-16 nm.
Similar sizes were also obtained for CeF3 NCs after they were synthesized by these methods.
In all synthesized NCs, a typical hexagonal-faceted crystalline structure was observed. The results indicate that all nanocrystals synthesis methods used in this study can enable a high control over the NC size in the range of 5-16 nm.
A clear microscopic image of charged patches, hydrophobic and hydrophilic composition, and surface dual properties of LnF3 NCs were obtained.
A direct comparison was established between the rod-like structures containing tilted NCs with a major part of their exposed surface conforming to nonmetal {1100} planes and the electrostatic response of flat-lying single NCs with an exposed metal-terminated {0001} plane.
A dual-faceted charge behavior was observed in the synthesized samples as planar NCs displayed a negative charge distribution owing to the exposed hexagonal metallic plane and rod NCs displayed a positive charge distribution owing to the interaction between the tip and nonmetal planes.
A considerable difference was observed in the surface potential between the two NC organizations.
The average surface potential on the planar individual NCs exposing {0001} planes was 280 mV more negative compared to the inclined NC assemblies dominated by {1100} planes. The results matched with the predictions based on all-atom MD simulations.
Taken together, the findings of this study established the basis for real-space visualization of patchy systems with the microscopic characterization of single NCs and demonstrated the feasibility of KPFM in detecting the presence of opposite charge patches in NCs.
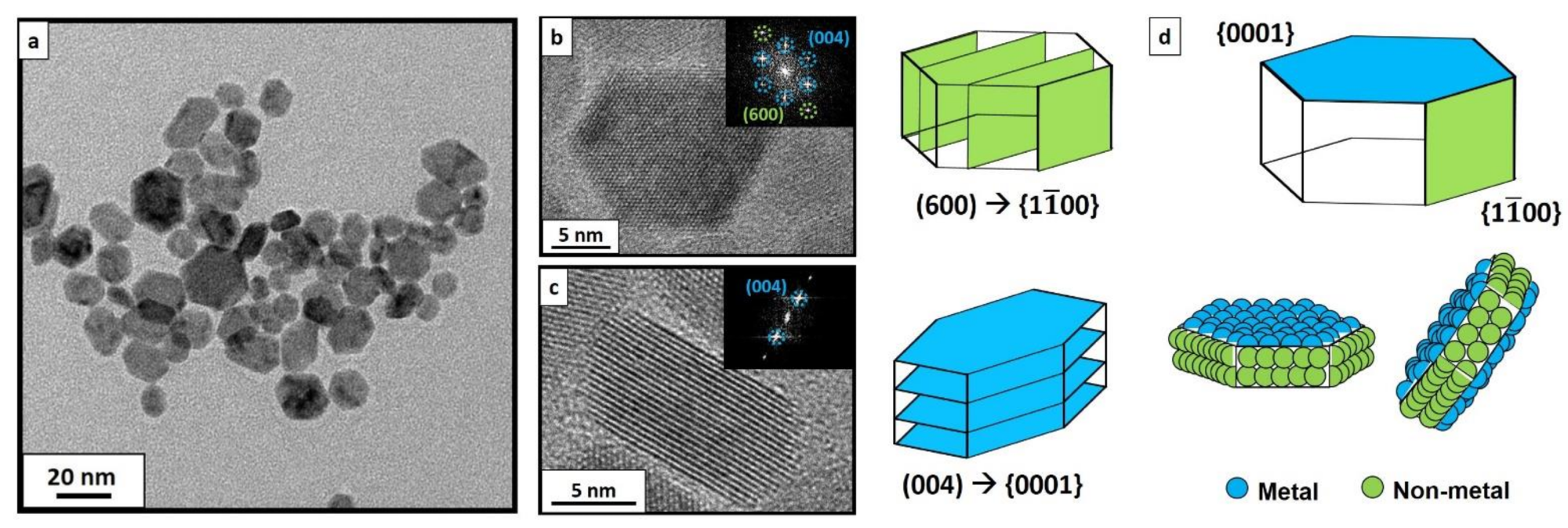
Figure 3. TEM image of CeF3 NCs synthesized via hydrothermal method at 210 °C (a). Well faceted-NCs could be observed tilted in several directions, randomly oriented. HRTEM images of as-synthesized CeF3 NCs via hydrothermal treatment at 210 °C with their corresponding faceting in {11¯00} (b) and in {0001} (c) planes. Deduced experimental faceting, as well as the exposed elements in each facet (d).© Martínez-Esaín, J., Pérez-Rodríguez, A., Faraudo, J. et al. (2022)
Reference
Martínez-Esaín, J., Pérez-Rodríguez, A., Faraudo, J. et al. (2022) Real-Space Image of Charged Patches in Tunable-Size Nanocrystals. materials 15, 1455. https://www.mdpi.com/1996-1944/15/4/1455
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.