A team of researchers published an article in the MDPI journal materials on untreated wastewater H2 fuel generation using Cu/CuO nanoporous electrodes. This is a novel approach to recycling polluted water.

Study: Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes. Image Credit: Peter Milto/Shutterstock.com
Cu/CuO nanoparticles were prepared by heating Cu conductive foil for 1 hour at 540 °C. The Cu/CuO photocatalytic activity generated 14.6 % H2 from sewage water.
Properties of light wavelength and intensity were also studied. The higher light intensity of 50 mWcm2 improved conductance from 2.2 to 4.7 mAcm2. The new study's results hold considerable potential for remote H2 fuel production.
Potential of H2 Gas Produced from Waste Water
Photocatalytic materials are a significant class of components with prospective uses in renewable-energy domains including solar cells, optoelectronics, and photocatalytic hydrogen generation.
On the one hand, producing hydrogen gas from untreated wastewater is a potential renewable energy industry. This method produces H2 gas, which may be used for a variety of everyday tasks such as heating and cooking, particularly in isolated and remote areas.
In addition to its typical usage in factory production and enterprises, this H2 gas is employed as a fuel for airplanes and aircraft. This photocatalytic process, on the other hand, eliminates pollution (sewage water) by converting it to fuel.
Furthermore, H2 is a clean energy source; its burning has no negative consequences. Utilizing renewable power minimizes the usage of fossil fuels, which emit harmful pollutants including CO2, and sulfur dioxide.
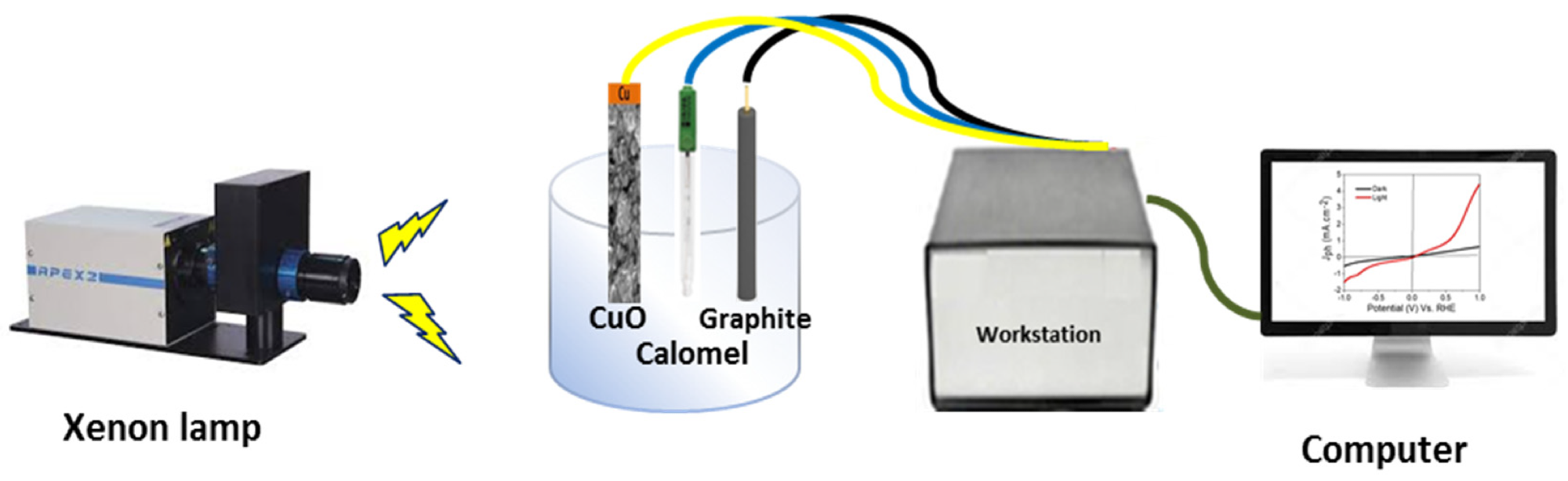
Figure 1. Schematic diagram for photoelectrochemical H2 generation process from sewage water. © Hadia, N., et al (2022)
Photocatalysts for H2 Production
To produce H2, highly conductive photocatalysts such as metal oxides, sulfides, nitrides, or organic materials must be utilized.
Due to their low cost, durability, and efficiency of fabrication, metal oxides are appealing photocatalyst materials for H2 production.
Photocatalytic efficiency may be improved by increasing surface area. Using photonic or thermally productive materials such as Cu metal to improve photocatalytic activity is also a possibility.
These materials successfully capture light and cause electron clustering activities across hybrid semiconductor materials, which are subsequently utilized to make H2 by adjacent materials.
Cu as a plasmonic material in Cu/ZnO was previously studied for light capture and photoelectrocatalytic amplification.
CuO and Cu2O as Potential Candidates for Clean Energy
CuO and Cu2O have absorption coefficient values of 0.7–1.5 eV and 2.0–2.3 eV, correspondingly, making them suitable materials for clean energy applications.
These materials' narrow bandgap characteristics allow them to collect the majority of light, which is preferred over wide bandgap semiconductors, which can only absorb 10% to 20% of sunlight.
Moreover, CuO's reduced bandgap and higher adsorption efficiency make it a good candidate for use in renewable energy (as opposed to Cu2O).
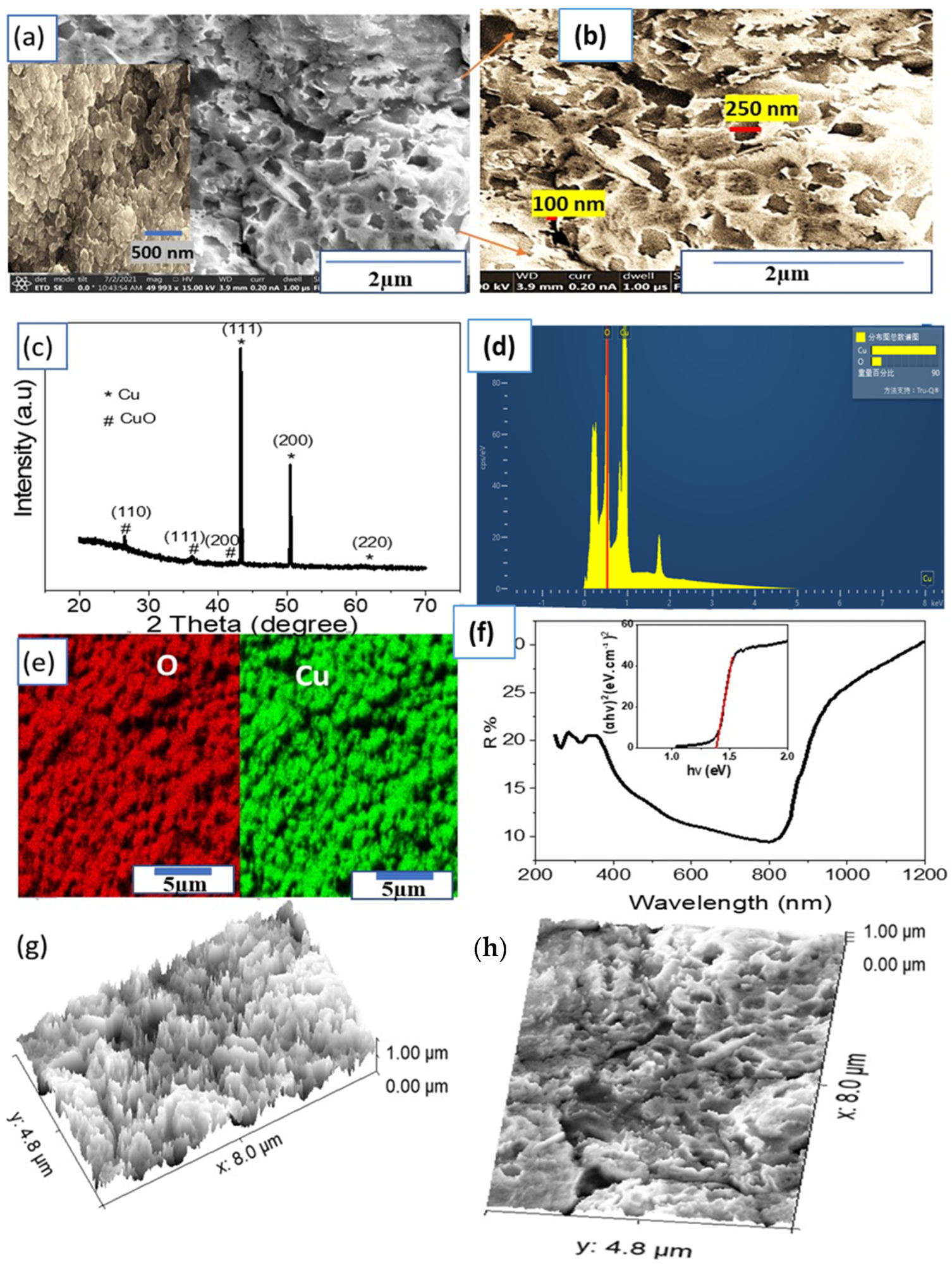
Figure 2. (a,b) FESEM images, (c) XRD pattern, (d) EDAX, (e) elemental mapping for O (red) and Cu (green), and (f) optical reflectance and bandgap (insert) of the CuO NP thin film. (g,h) The cross section and surface roughness using the modeling program (ImageJ), respectively. © Hadia, N., et al (2022)
Limitations of CuO for Energy Applications
The majority of prior CuO research depended on the inclusion of a sacrificial reagent such as NaOH, Na2S2O3, or HCl. Furthermore, the H2 rate of production was restricted.
Chemical blasting, physical vapor deposition, spray pyrolysis, RF sputtering, and other techniques for fabricating CuO have all been documented. These approaches have limitations such as significant expenses, extended production times, and complicated fabrication procedures.
Production of H2 Gas Fuel from the Waste Water
Researchers reported producing H2 gas from sewage water without requiring any external reagent.
The catalyst electrode is made utilizing a simple, low-cost combustion approach. Comparatively, the constructed electrode has a substantial Jph. This electrode's effectiveness is intriguing for industrial H2 production from untreated wastewater.
A Cu/CuO photocathode made by oxidation/combustion was assessed for H2 generation from sewage water splitting without a sacrificial agent. The effect of light wavelength and intensity, heat, and on/off chopping amperage on photocathode performance was studied by researchers.
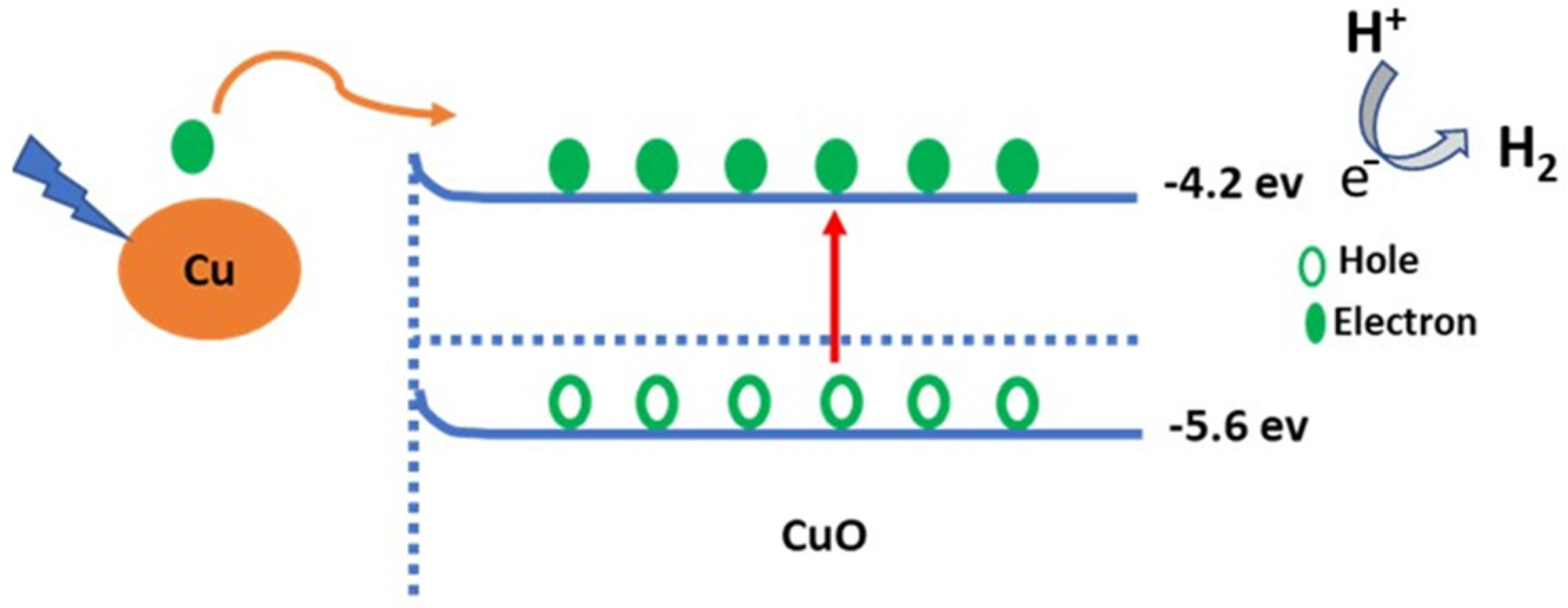
Figure 3. Schematic diagram of the sewage water splitting mechanism © Hadia, N., et al (2022)
Conclusions
Using a CuO NP photoelectrode to generate H2 from wastewater shows promising results. The investigations were all done to confirm the CuO NP's chemical composition, shape, and photocatalytic activity.
Overall, the team was able to demonstrate that the Cu/CuO photoelectrode produced H2 gas from sewage water.
Reference
Hadia, N., Abdelazeez, A., Alzaid, M., Shaban, M., Mohamed, S., Hoex, B., Hajjiah, A. and Rabia, M., (2022) Converting Sewage Water into H2 Fuel Gas Using Cu/CuO Nanoporous Photocatalytic Electrodes. Materials, 15(4), p.1489. https://www.mdpi.com/1996-1944/15/4/1489
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.