In a pre-proof paper available in the journal Biochemical and Biophysical Research Communications researchers demonstrated the key role played by the geometry of graphene oxide (GO) nanomaterials, such as quantum dots and nanosheets, in influencing the biophysical impacts on hepatic stellate cells (HSCs).
Study: The geometry-dependent regulation of hepatic stellate cells by graphene oxide nanomaterials. Image Credit: Jose Luis Calvo/Shutterstock.com
Graphene Oxide in Biomedical Applications
Nanomaterials are used extensively in biomedical applications such as photothermal therapy, bioimaging, and drug delivery. Specifically, graphene oxide (GO) nanomaterials are one of the most preferred drug delivery methods for the treatment of liver diseases owing to their biocompatibility and tunable physical and chemical properties. GO quantum dots and nanosheets are the common GO geometric forms used in biomedical applications.
However, a significant share of administered nanoparticles is often accumulated in livers for a considerable duration. The time needed for their clearance ranges between 1 and 21 days, based on the different physiochemical properties of nanomaterials. This long retention time and slow clearance can lead to chronic toxicity to livers and cause liver diseases in the long term, such as liver fibrosis.
Liver fibrosis is primarily triggered by severe liver injury caused by metabolic disorders or viral diseases. The activation of HSCs, one of the major fibrogenic cells in the liver that are located between hepatocytes and endothelial cells in liver sinusoid, represents the initial disease development stage of liver fibrosis.
Although different interactions between the other major types of liver cells and nanomaterials were investigated extensively in previous studies, only a few studies investigated the interaction between HSCs and nanomaterials.
Here, it was reported that nanomaterials can alter cell morphology in rat HSCs and cause toxicity to human HSCs. For instance, GO and reduced GO flakes at concentrations greater than 31.25 μg/ml inhibited human HSC growth.
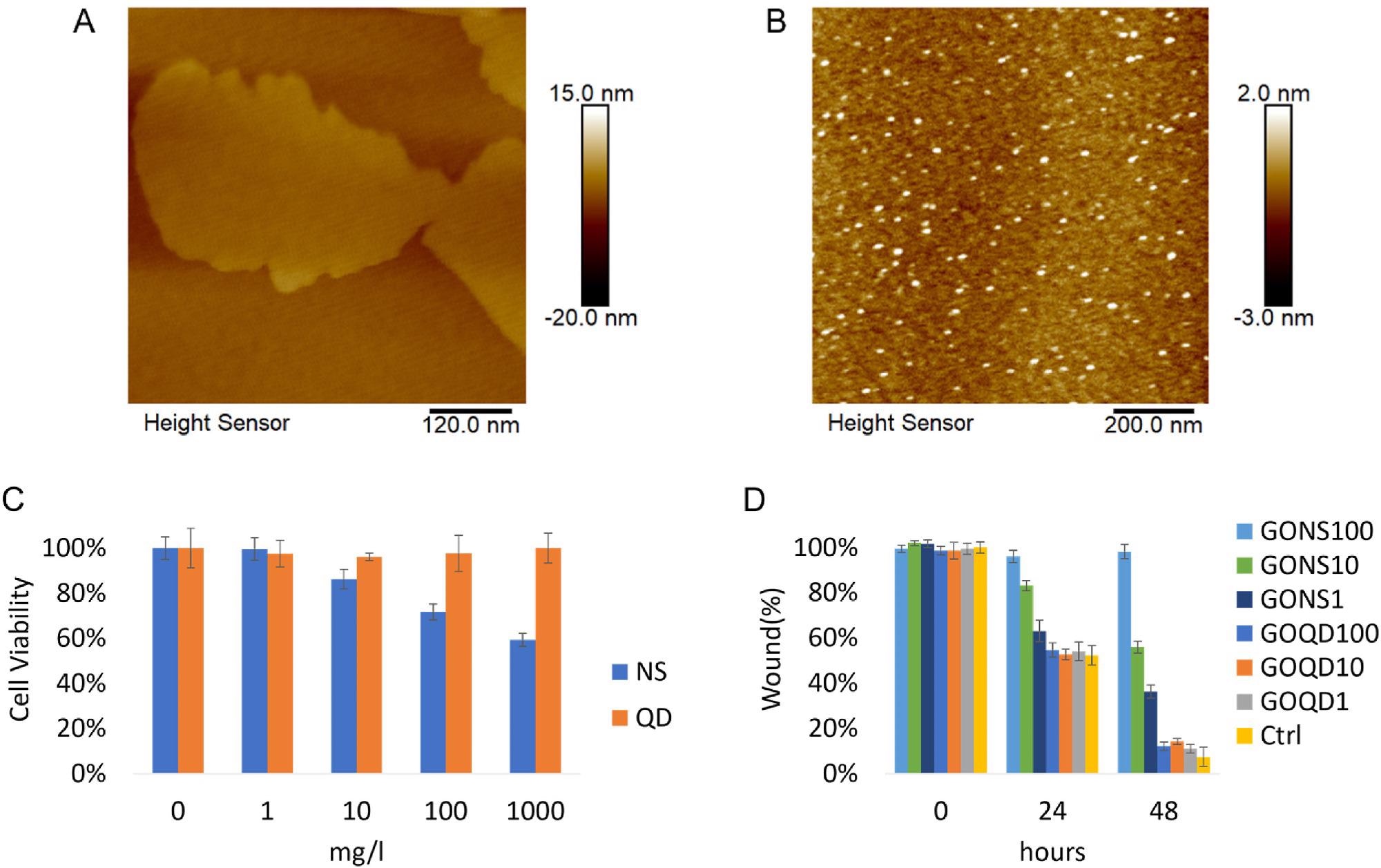
Figure 1. A) Representative AFM image of GO nanosheets. B) Representative AFM image of GO quantum dots. C) Cell viability of LX-2 incubated with GO nanosheets and quantum dots were assessed with CCK-8 assay after 24 h incubation. (*p < 0.05) D) The evaluation of GO nanomaterials in affecting activated LX-2 mobility. Cells were treated with GO nanosheets and quantum dots ranging from 1 to 100 mg/l (NS: nanosheets, QD: quantum dots. Ctrl: control). © Gui, X., Hu, G., Jie, Z. et al. (2022)
Effects of GO Nanomaterials on HSCs
In this study, researchers investigated and compared the geometric effects of GO quantum dots and nanosheets on human HSCs in terms of regulation pathways, mobility, fibrotic degree, and cell viability to understand the geometry-dependent impact of nanomaterials, as well as their underlying mechanisms.
A JC-1 assay kit and enhanced chemiluminescence kit were employed to perform the investigations. A Zetasizer Nano-Z was utilized to determine the zeta potentials of the GO quantum dots and nanosheets, while their thickness, size, and geometry were characterized using an atomic force microscope (AFM).
LX-2 cells were grown in DMEM with 100 μg/ml streptomycin, 100 U/ml penicillin, and 10% fetal bovine serum, and then maintained in an incubator with the humified atmosphere at 37oC and 5% carbon dioxide to perform cell culture.
In the mobility assay, a microscope was used to obtain the cell images at 48, 24, and 0 hours, and the distance of migration was measured and statistically analyzed by LAS X software and analysis of variance (ANOVA), respectively.
4′,6-diamidino-2-phenylindole (DAPI) was utilized to stain the LX-2 cell nuclei during fluorescence cell staining, while a confocal microscope with 63x oil immersion objective was employed to obtain images during confocal microscopy analysis.
Transmission electron microscope (TEM) analysis was performed to evaluate the geometry-dependent subcellular effect, while the GO nanomaterial effects on the cell mitochondrial membrane potential were investigated by a fluorescence microscope using the JC-1 assay kit. An Amersham ImageQuant 800 was employed to obtain images during the western blot test.
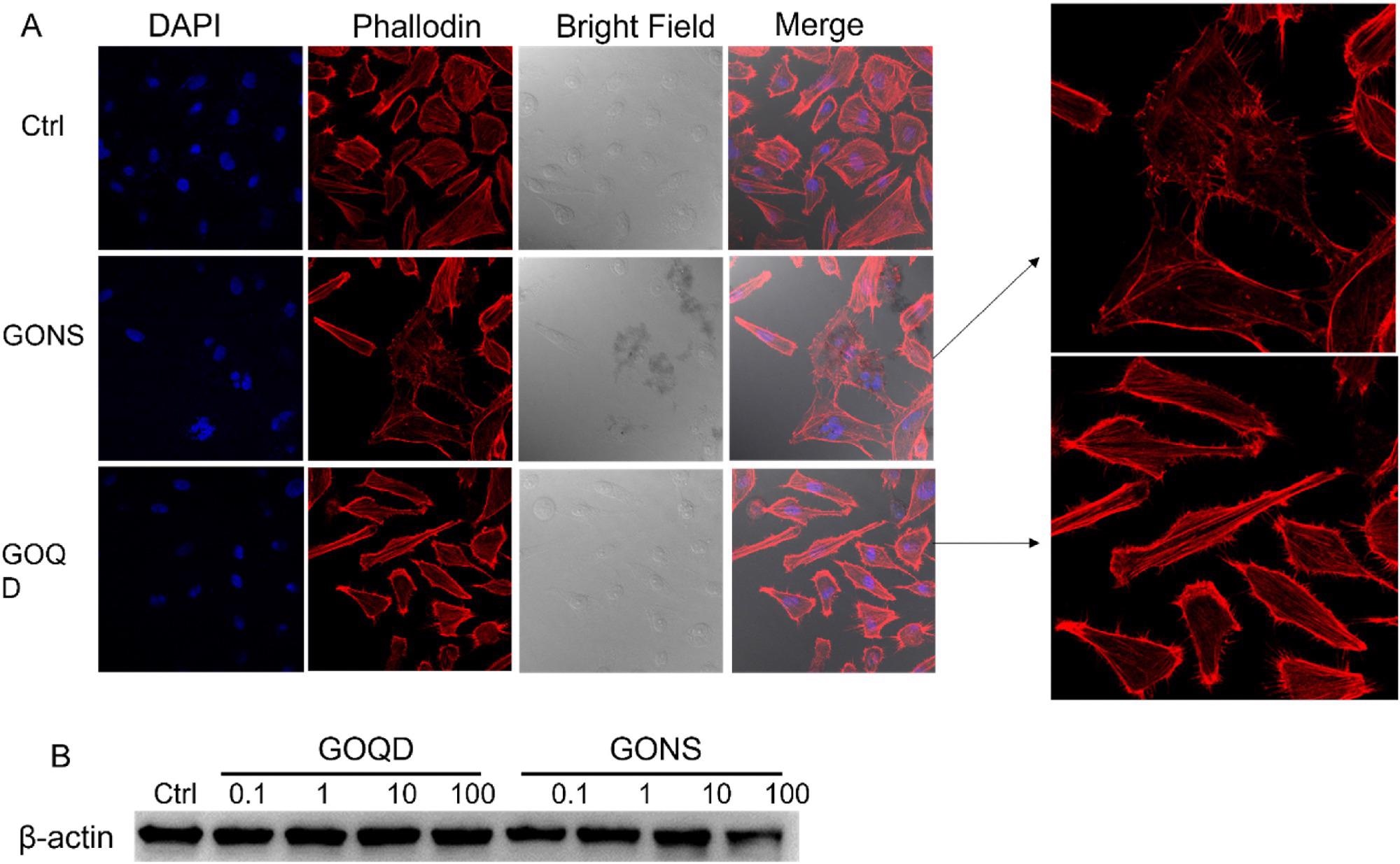
Figure 2. A) Morphology changes of induced by GONS and GOQD observed by confocal microscope. B) Western blot detecting β-actin expression level when LX-2 incubated with GO quantum dots and nanosheets ranging from 0.1 to 100 m/l for 24 h. The expression level of β-actin decreased after LX-2 incubated with 100 mg/l GO nanosheets. © Gui, X., Hu, G., Jie, Z. et al. (2022)
Observations
The thickness of the GO nanosheets and quantum dots were 4 nm and 2 nm, while the zeta potential was -29.6 ± 1.3 mV and -2.1 ± 0.9 mV, respectively.
GO nanosheets significantly reduced the cell mobility and viability of HSCs. Protein expression levels of Smad3/Smad2/TGFβR reduced corresponding with the attenuating fibrotic degree. However, the protein expression level of α-SMA, which plays a key role in liver fibrosis, increased.
At 100 mg/l concentration, GO quantum dots reduced the GADPH and α-SMA expression levels, while the β-actin expression levels remained constant. Mitochondrion analysis showed that GO nanosheets disrupted the membrane and membrane potentials of mitochondria.
The nanosheets activated HSCs and induced oxidative stress through the reactive oxygen species pathway while modulating the fibrotic effect through the TGF-β pathway. The observation was verified by the reduced α-SMA expression level after co-incubation of n-acetyl cysteine and GO nanosheets with HSCs.
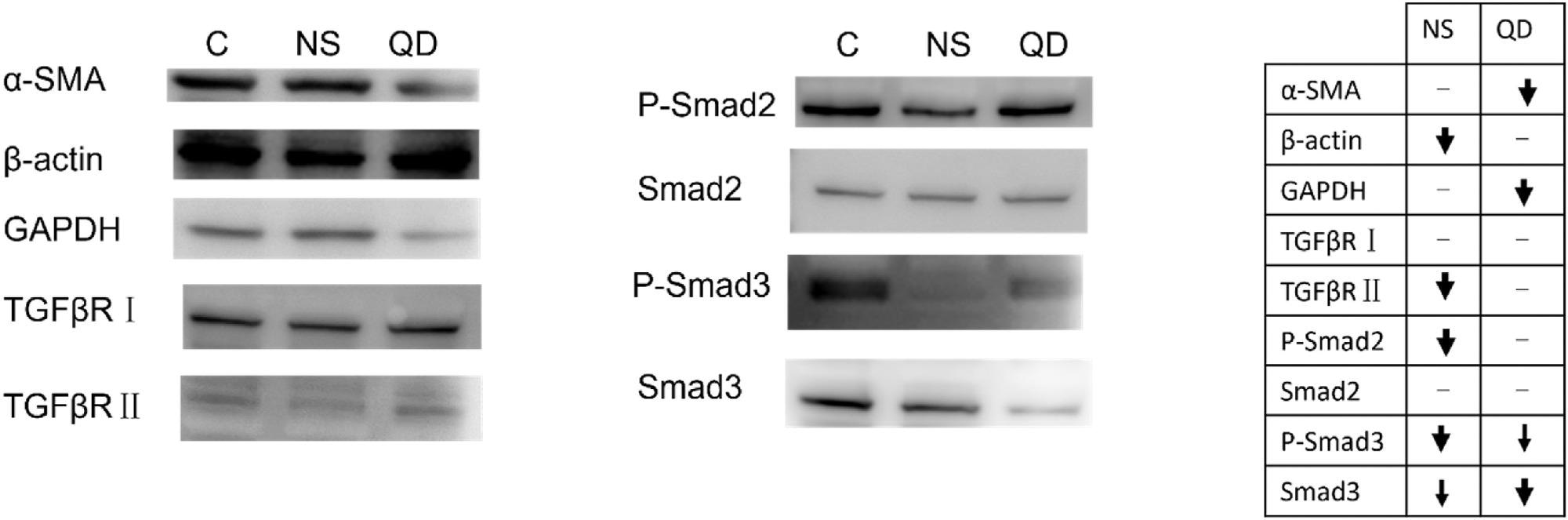
Figure 3. Western blot evaluation of proteins potentially involved in geometry-dependent regulation of LX-2. In the chart, bolder arrows denote a more significant change in expression level. (C: control, NS: GO nanosheets, QD: GO quantum dots). © Gui, X., Hu, G., Jie, Z. et al. (2022)
Taken together, the findings of this study demonstrated that the geometry of the GO nanomaterials plays a significant role in different biochemical and biophysical responses from the HSC cells, which can potentially help in developing anti-fibrotic therapy with rGO nanosheets and GO quantum dots. However, more research is required to explore the correlation between α-SMA and GADPH.
Reference
Gui, X., Hu, G., Jie, Z. et al. (2022) The geometry-dependent regulation of hepatic stellate cells by graphene oxide nanomaterials. Biochemical and Biophysical Research Communications https://www.sciencedirect.com/science/article/pii/S0006291X22003758?via%3Dihub
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.