In an article recently published in the open-access MDPI journal nanomaterials, researchers investigated the mechanism of interaction between nanoscale materials and specific cells for biomedical applications, primarily related to targeted delivery and tracing.
Study: Cellular Uptake of Gold Nanorods in Breast Cancer Cell Lines. Image Credit: David A Litman/Shutterstock.com
Here, the localization, cellular uptake quantity, and response time of gold nanorods in MCF-7 and SKBR-3 breast cancer cells were measured.
Background
The amalgamation of biotechnology and nanotechnology has been a quite fascinating field of research for the last few years.
Currently, nanomaterials are extensively used in biomedical applications such as drug delivery, magnetic or fluorescent bio-imaging, tissue engineering, tracing pathogens in sensitive organs, cancer chemotherapy, and many more. However, each nanomaterial interacts differently with a particular type of cell.
The specific combinations of nanomaterials and cells with varying shape, size, and surface chemistry is a vast domain of research. Thus, it is crucial to understand the mechanisms of all possible types of interactions between nanomaterials and cells.
Many studies have shown cellular uptake of gold nanorods by cancer cells. MCF-7 and SKBR-3 cancer cell lines represent HER2-negative and HER2-positive breast cancer models, respectively. These cells intake gold nanorods through a mechanism occurring at the surface of the cell membrane, called endocytosis.
Inductively coupled plasma mass spectrometry (ICP-MS) can measure the efficiency of targeted delivery and cellular uptake of these nanoparticles, while transmission electron microscopy (TEM) can be used to investigate their localization.
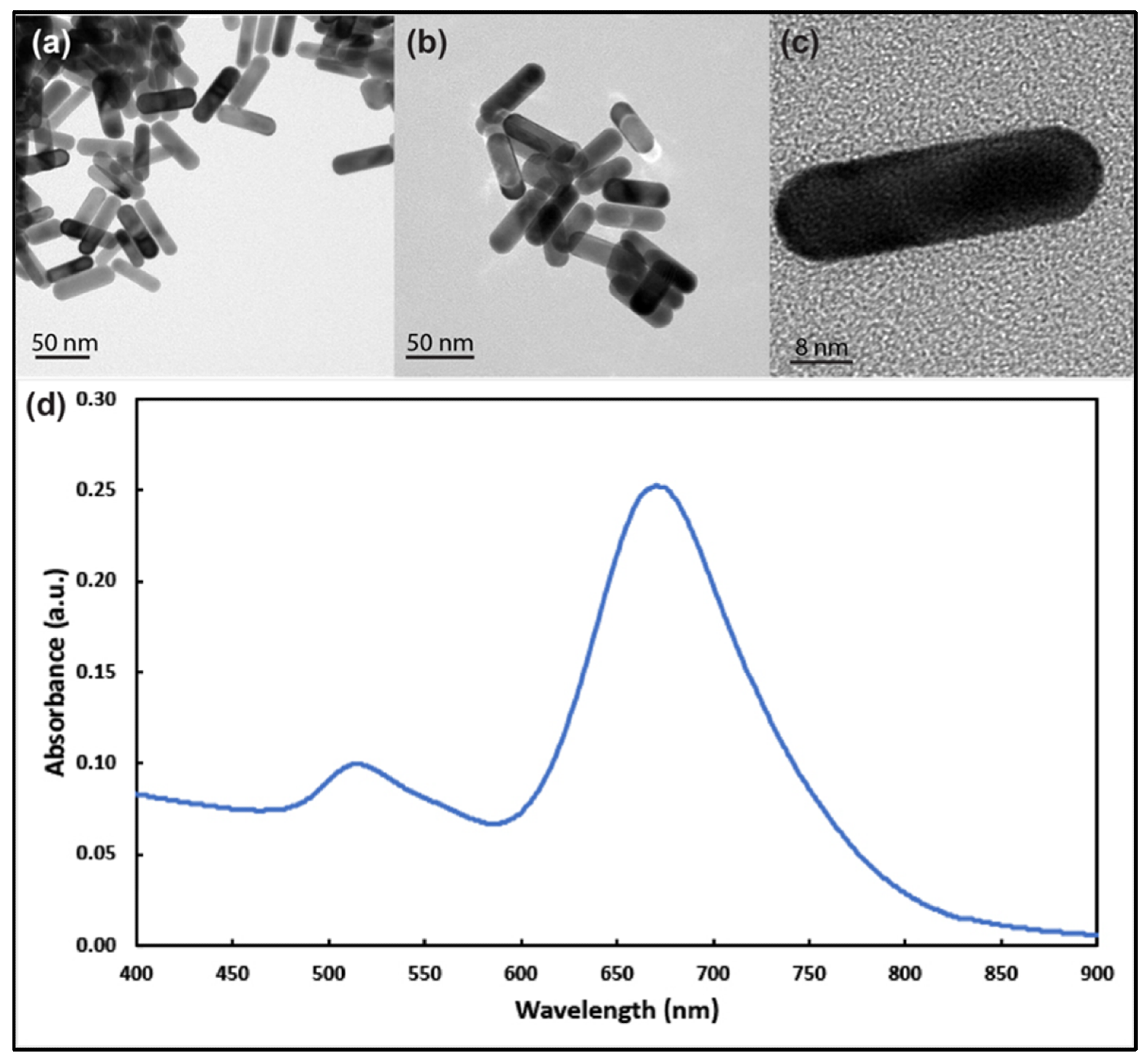
Figure 1. Transmission electron microscope images of gold nanorods with aspect ratio around 3 at different magnifications (a–c). (d) UV–visible spectrum analysis of the prepared gold nanorods showing longitudinal and transverse plasmons around 690 nm and 514 nm, respectively. © White, B., White, M., Nima Alsudani, Z., Watanabe, F., Biris, A., Ali, N., (2022)
About the Study
In this article, researchers investigated the interaction mechanism of gold nanorods (Au NRs) in two types of breast cancer cell lines namely, MCF-7 and SKBR-3. More than 200 Au NRs with an average length and diameter of 36 and 12 nm, respectively, were synthesized using the silver ion-assisted seed-mediated method. Subsequently, MCF-7 and SKBR-3 cells were cultured in a conducive medium and allowed to bind with different concentrations of Au NRs for 1 and 2 days.
The excess Au NRs were washed away from the treated cancels using phosphate buffer saline (PBS) solution followed by treating with the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) reagent. Finally, ICP-MS and TEM analyses were performed to investigate the interaction mechanism.
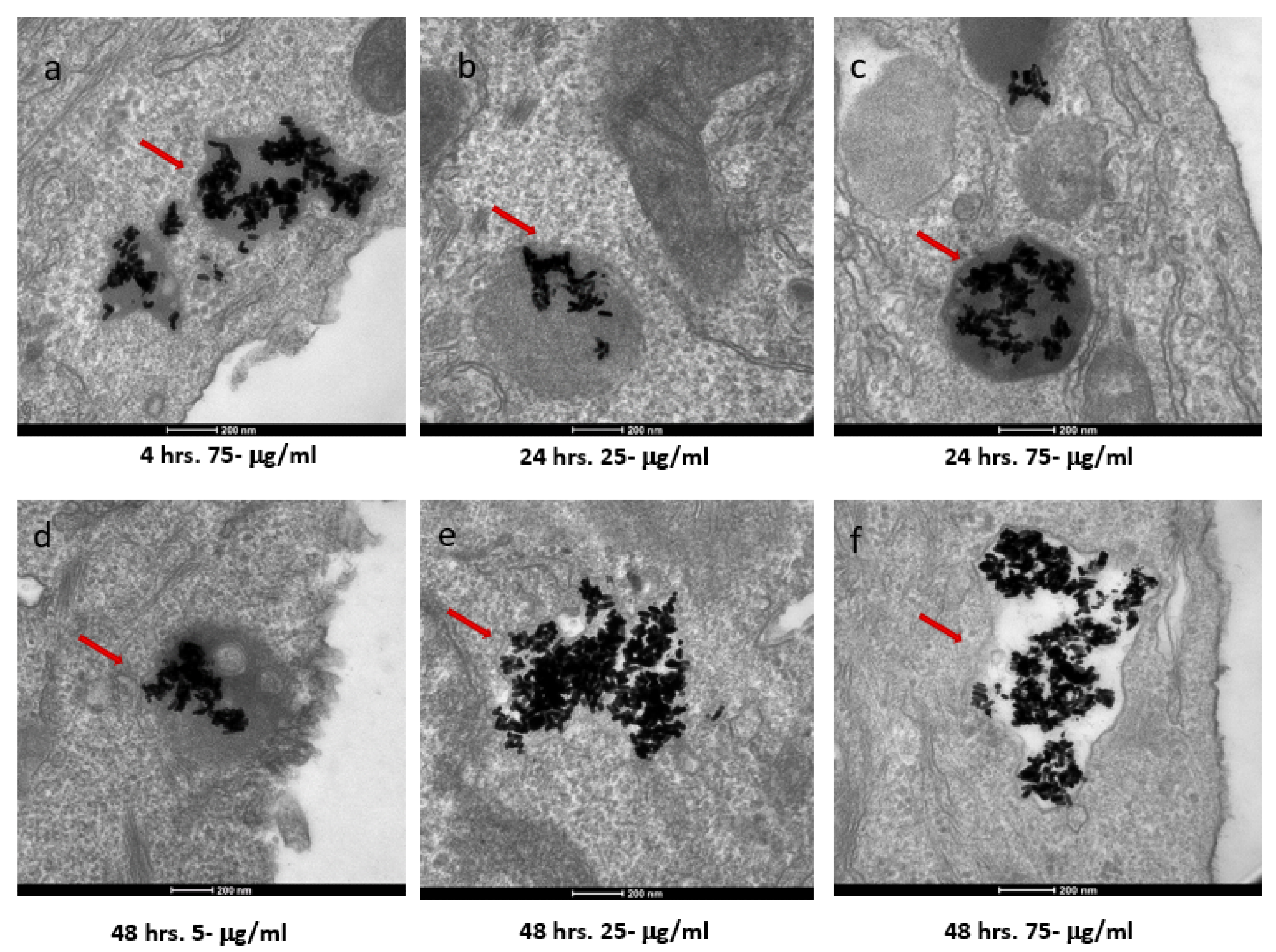
Figure 2. TEM images of AuNRs in SKBR-3 cells at different time points and concentrations. SKBR-3 cells were treated with 5, 25, and 75 μg/mL of AuNRs for 1, 4, 24, and 48 h. Internalization of nanorods was observed starting at 4hourincubation with 75 μg/mL (a). After 24hourof incubation, internalization was observed with the 25 μg/mL (b) and 75 μg/mL concentrations (c). For cells incubated for 48 h, we observed internalization with 5, 25, and 75 μg/mL concentrations (d, e, and f, respectively). Red arrows point to gold nanorods localized in SKBR-3 cells. Electron photomicrographs shown are representative of at least three independent experiments with similar conditions. © White, B., White, M., Nima Alsudani, Z., Watanabe, F., Biris, A., Ali, N., (2022)
Observations
TEM images showed Au NRs had longitudinal and transverse plasmon resonances at around 680 and 514 nm, respectively. The average zeta potential of the NRs was −47.18 ± 4.26 mV. Au NRs did not show any cytotoxicity in healthy human cells. MTT methods indicated that the MCF-7 cells were less sensitive to Au NR exposure than the SKBR-3 cells for longer exposure times at higher concentrations. The SKBR-3 cells exhibited a drop in cell vitality due to higher sensitivity, whereas the MCF-7 cells had almost no drop in cell viability.
Both types of cells demonstrated a different pattern in cellular uptake of Au NRs for different exposure times and concentrations. The SKBR-3 cells had a constant cellular uptake between one to four hours, but 25 μg/mL Au NRs concentration had a higher uptake than the 75 μg/mL sample, i.e. lower concentration had a higher cellular uptake. In contrast, in MCF-7 cells, the 75 μg/mL AuNR-treated cell samples exhibited higher cellular uptake than 5 and 25 μg/mL samples, i.e. higher concentration samples had higher cellular uptake than lower concentration samples.
Furthermore, TEM analysis showed that the Au NRs interacted with the cell membrane of both cancer cells starting from one to 48 hours of exposure time. However, the Au NRs entered into the cell (i.e. internalized) for the samples with concentrations of 25 and 75 μg/mL. The 5 μg/mL concentration samples were never internalized. Moreover, the minimum exposure time for internalization was one hour and four hours for the MCF-7 and SKBR-3 cells, respectively.
The mechanism for the cellular uptake of the Au NRs was primarily micropinocytosis instead of receptor-mediated endocytosis. These Au NRs-carrying macropinosomes further interacted with the lysosomes inside the cells.
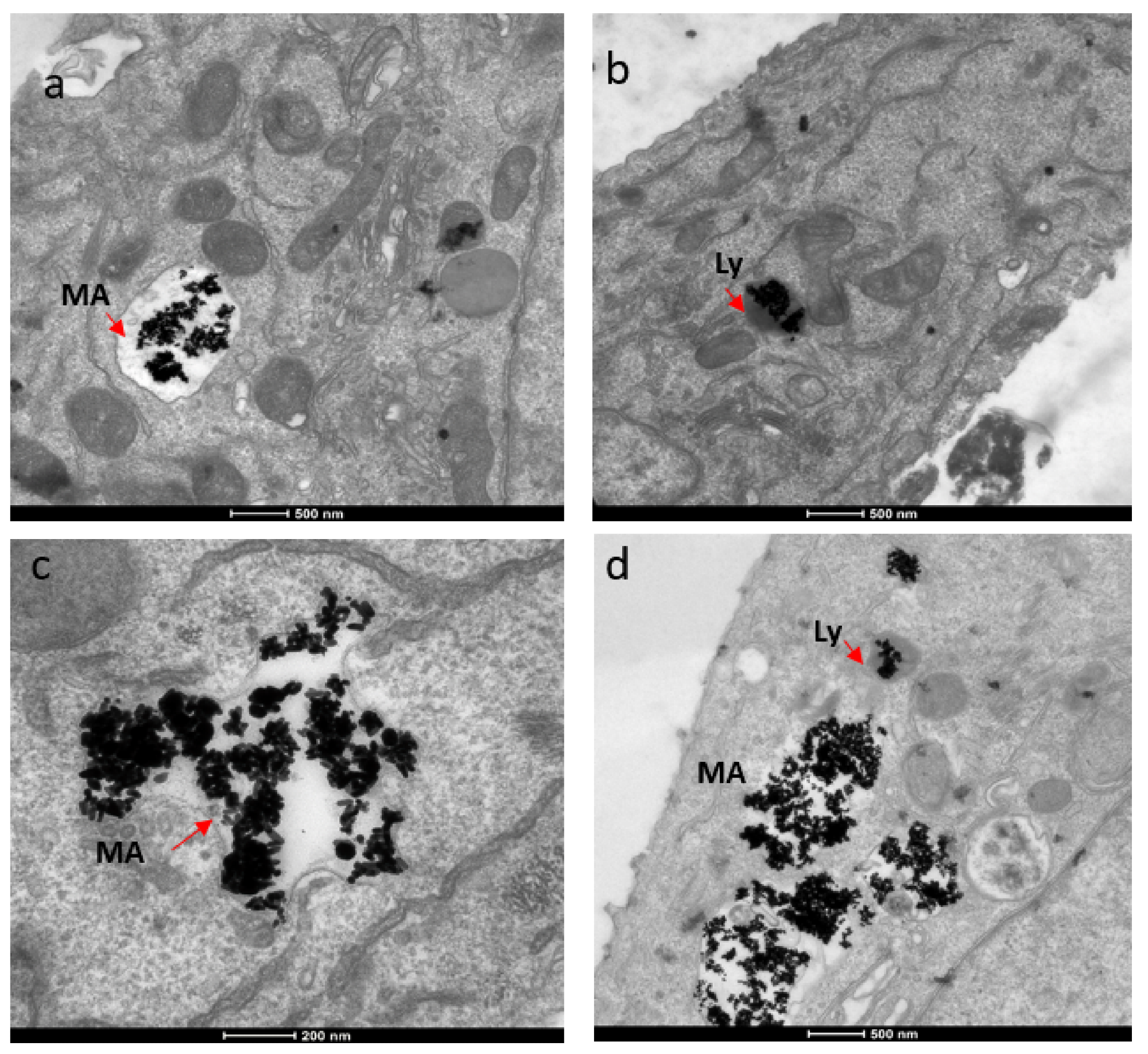
Figure 3. TEM images showing localization of gold nanorods in macropinosomes and lysosomes in both SKBR-3 and MCF-7 cell lines. AuNR localization in (a) macropinosomes (MA) and (b) lysosomes (Ly) of SKBR-3 cells. (c) Macropinosomes (MA) containing gold nanorods in MCF-7 and (d) lysosomes (Ly) containing gold nanorods in MCF-7. Red arrows point out macropinosomes and lysosomes containing gold nanorods in each image. Electron photomicrographs shown are representative of at least three independent experiments with similar conditions. © White, B., White, M., Nima Alsudani, Z., Watanabe, F., Biris, A., Ali, N., (2022)
Conclusions
To conclude, the researchers of this study investigated the interaction of Au NRs in two types of breast cancer cell lines, i.e. MCF-7 and SKBR-3. The shape, size, and zeta potential of Au NRs had different effects on cell vitality and toxicity of different cancer cells. Additionally, both cell types had different patterns of cellular uptake for Au NRs at different concentrations and exposure times.
The Au NRs did not enter into the cancer cells for samples with lower concentrations of NRs or lower exposure time. Also, the cellular uptake mechanism was primarily driven by micropinocytosis. These findings will help improve biomedical applications related to targeted drug delivery, tracing, and bio-imaging involving nanomaterials.
Reference
White, B., White, M., Nima Alsudani, Z., Watanabe, F., Biris, A., Ali, N., (2022) Cellular Uptake of Gold Nanorods in Breast Cancer Cell Lines. Nanomaterials, 12, 937. https://www.mdpi.com/2079-4991/12/6/937
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.