A paper recently published in the journal ACS Omega reviewed the current status and potential of X-ray spectroptychography for the characterization of advanced nanomaterials.

Study: X-ray Spectroptychography. Image Credit: Gorodenkoff/Shutterstock.com
What is X-ray Spectroptychography?
X-ray spectroptychography is an emerging technique that represents the ptychographic version of X-ray spectromicroscopy and is used for the chemical microanalysis of nanomaterials such as batteries and catalysts. This technique is based on the existing synchrotron X-ray spectromicroscopy and microscopy techniques with an added ptychography, an algorithmic imaging technique, for image acquisition.
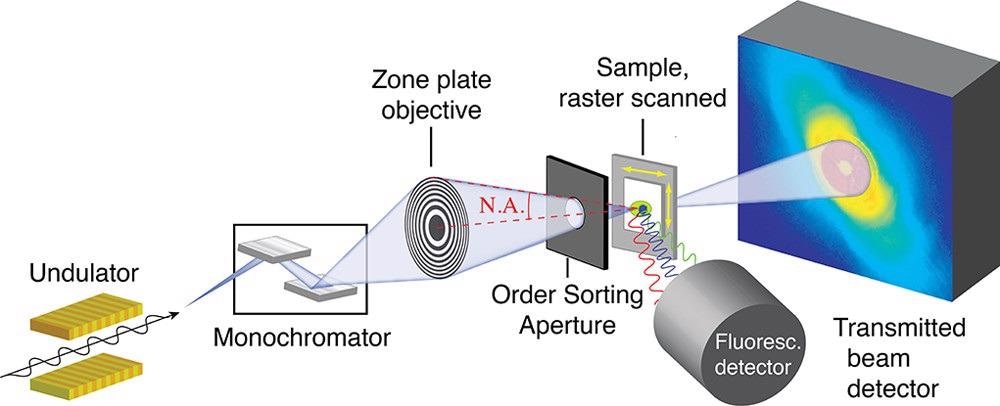
Figure 1. Schematic of a scanning transmission X-ray microscope. Adapted with permission from ref (45). Sample is raster scanned through the focused X-ray beam. In conventional STXM, transmission is measured by a serial detector downstream of the sample. In ptychographic STXM, a diffraction pattern is measured with a pixelated detector downstream of the sample, as shown in the figure. © Urquhart (2022)
X-ray spectroptychography provides a higher spatial resolution during chemical microanalysis compared to conventional X-ray optics. In traditional X-ray microscopes, the spatial resolution is limited by the focusing properties of their X-ray optics. However, ptychography can significantly enhance the spatial resolution of X-ray microscopy as this lensless imaging technique relies on the diffraction of coherent radiation by an amorphous sample.
Within coherent diffractive imaging (CDI), the forerunner of ptychography, the reconstruction of the image of an isolated object is based on the diffraction pattern of the object, which is obtained by the uniform illumination of the object. This factor limits the practical application of the technique. However, unlike the CDI method, ptychography utilizes a sequence of coherent far-field diffraction patterns obtained from the overlapping regions of the isolated sample without requiring the sample.
Ptychography microscopes use zone plates, refractive Laue lenses, Kirkpatrick−Baez mirrors, or pinholes to illuminate the test sample with a well-defined coherent probe. These optics provide a constant focal length when the X-ray energy is altered for spectroscopy.
Both phase and absorption can be utilized for ptychography image contrast. Phase contrast is more crucial in a hard X-ray measurement obtained from a test sample. X-ray spectroptychography can provide the full refractive index of a sample comprising the phase and absorption spectra.
X-Ray Spectroptychography Applications
In the last few years, the spatial resolution provided by X-ray spectroptychography was enhanced owing to the improvements in algorithms and instrumentation associated with the technique. Currently, the technique is used to study nanostructured energy materials such as magnetic materials and fuel cell cathodes.
Magnetic Materials
X-ray magnetic circular dichroism (XMCD) spectroptychography can be used to evaluate the magnetic nanostructure of materials. For instance, XMCD spectroptychography was used to investigate the out-of-plane magnetization in gadolinium/iron multilayer samples and study the nanoscale magnetite single crystals synthesized by magnetotactic bacteria.
Battery Materials
X-ray spectroptychography can provide a chemical characterization of battery materials at a higher spatial resolution compared to conventional X-ray spectromicroscopy and microscopy. Specifically, this technique provides an oxidation state mapping of particular metal ions with a high spatial resolution during battery cycles. For instance, iron L3-edge X-ray spectroptychography was used to map delithiation and lithiation through the corresponding changes in the iron 2p oxidation state.
The metal oxidation state was also mapped at hard X-ray energies using this technique. Spectroptychography is used for chemical mapping to monitor the relationship between the chemical and mechanical stability of lithium-ion batteries. The technique is also used for the elucidation of surface chemistry and minority phases.
Catalyst Materials
X-ray spectroptychography is used extensively to investigate catalyst materials as the improvement of these materials depends on high spatial resolution chemical mapping of heterogeneous materials. Additionally, this technique is also used to evaluate porosity in heterogeneous catalyst materials such as fluid catalytic cracking catalysts.
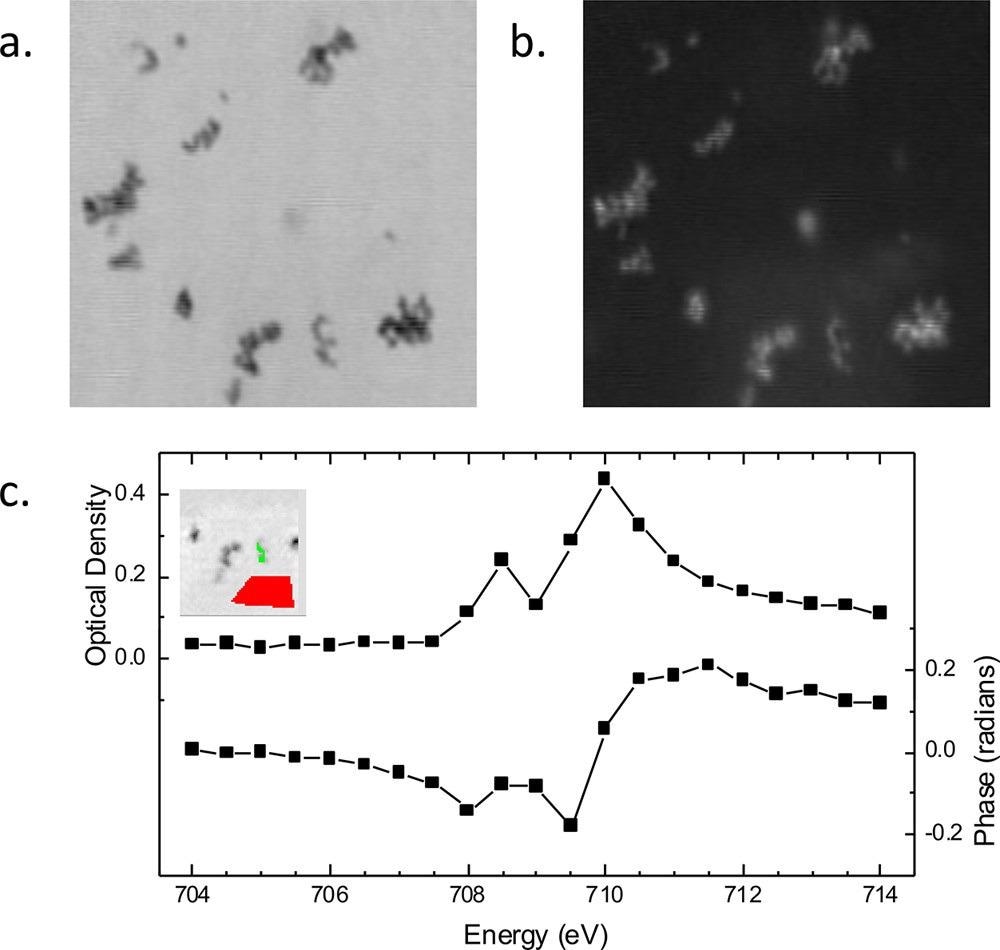
Figure 2. Spectroptychography of 30 nm diameter Fe2O3 nanoparticles. Amplitude (a) and phase (b) ptychography images, recorded at 710.0 eV. Image size is 2.1 × 2.1 μm. (c) Absorption (optical density) and phase Fe L3 spectra obtained from this sample. (Inset) Region from which the sample signal for amplitude and phase (green region) was extracted. Amplitude signal from an open area (red region) was used for the incidence flux in the calculation of the sample optical density using Beer’s law, −ln(I/Io). © Urquhart (2022)
Other Aspects Related to X-ray Spectroptychography
Spectroptychotomography
Three-dimensional (3D) mapping using spectroptychotomography can help in avoiding spurious correlations in two-dimensional (2D) transmission imaging. For instance, a “sparse” spectroptychotomography study based on the simultaneous algebraic reconstruction technique (SART) was used to reduce the number of projections needed to obtain a high-quality reconstruction.
Although multi-energy spectroptychotomography studies can provide chemical mapping in a detailed manner, these studies are often restricted to radiation-resistant materials and are extremely time-consuming.
Extended X-ray Absorption Fine Structure (EXAFS) Spectroptychography
EXAFS spectroptychography requires several photon energies to provide an interferogram, which can be then Fourier transformed to obtain a radial distribution function. However, EXAFS spectroptychography studies require excellent stability and are time-consuming.
In Situ and Operando Spectroptychography
This technique can be potentially used to investigate samples in realistic and varied conditions. However, the dwell times needed for ptychography measurements and the requirement for several energies for spectroscopic sensitivity are the major challenges of in situ studies.
X-ray Linear Dichroism Spectroptychography
This technique can be used in several applications. For instance, the linear dichroism of a vanadium pentoxide crystal was investigated by X-ray linear dichroism spectroptychography at orthogonal polarization states and the V K edge to obtain phase and absorption maps of polycrystalline vanadium pentoxide.
Future of X-ray Spectroptychography
X-ray spectroptychography and ptychography are rapidly becoming the preferred synchrotron techniques for optics and instrumentation. However, the phase spectra and images of materials with complex structures obtained by these techniques cannot be easily rationalized, which is a major drawback. Phase spectra can reveal additional sample-related information, while phase images can be used to a limited extent for chemical imaging of samples with decreased radiation damage.
Additionally, X-ray cameras display reduced sensitivity at lower photon energies, which limits the application of spectroptychography in different materials such as lithium-ion batteries and organic electronic batteries. A more consistent signal-to-noise ratio is required to obtain higher spatial resolution measurements. Recent studies have demonstrated that spectroptychography has significant potential for correlative imaging when it is used with other microscopy techniques.
Complex ptychographic data sets can be analyzed using statistical methods such as machine learning, which can help in the translation of advanced statistical analysis and reconstruction methods to experimentally accessible tools.
To summarize, X-ray spectroptychography is fast becoming a mainstream method for the chemical microanalysis of nanomaterials. However, the time required for data acquisition remains a challenge in this technique.
Reference
Urquhart, S.G. (2022) X‑ray Spectroptychography. ACS Omega. https://pubs.acs.org/doi/10.1021/acsomega.2c00228
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.