 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Apr 7 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Apr 7 2022In an article recently published in the open-access journal ACS Omega, researchers described a unique technique for protecting murals with graphene-based nanomaterials.

Study: Polyacrylic Acid-Functionalized Graphene@Ca(OH)2 Nanocomposites for Mural Protection. Image Credit: salajean/Shutterstock.com
The team developed a facile and cost-effective aqueous process to strategically synthesize a polyacrylic acid-functionalized graphene/nano-Ca(OH)2 (PAAG@Ca(OH)2) material.
What are Murals?
Murals are an essential component of humanity's rich cultural legacy. They are, however, prone to serious damage over time, such as hollowing, fungus damage, flaking, and cracking. As a result, the development of mural reinforcing materials is becoming increasingly important for cultural heritage preservation.
Mural reinforcing materials are primarily split into two categories: inorganic and organic.
The use of inorganic materials will inevitably affect the aqueous solution in the mural, resulting in the crystallization of the soluble salts and poor permeability. Hence, organic acrylic polymers have recently been used to strengthen murals due to their outstanding adhesion, permeation, and transparency.
However, after heat oxidation and optical irradiation, organic polymer materials develop a noticeable aging problem, resulting in mechanical strength reduction.

Importance of Graphene Nanomaterials in Murals
The distinctive nanoparticle size and physicochemical features of diverse nanomaterials have gained attention in protecting heritage. Graphene's unique structure and composition have led to its integrtion across many sectors, yet its involvement in historical preservation is small, especially in mural applications.
Polyacrylic Acid-Functionalized Reduced Graphene Oxide (PAAG)@Ca(OH)2 Nanocomposite for Protection of Murals
In the present study polyacrylic acid-functionalized graphene was combined with nano-Ca(OH)2 to form a PAAG@Ca(OH)2 nanocomposite. The PAAG@Ca(OH)2 nanocomposites were manufactured using a cost-effective, facile, aqueous approach for mural protection using polyacrylic, acid-functionalized graphene.
The Hummers method was used to create graphite oxides from natural graphite powder to manufacture graphene oxide (GO). To better comprehend the PAAG@Ca(OH)2 nanocomposites, a high-resolution transmission electron microscopy (HRTEM) image was examined.
The (101) crystal plane of the Ca(OH)2 phase was assigned a lattice distance of 0.263 nm, which was compatible with the SAED pattern of the nanocomposites. The elemental mapping and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images of the PAAG@Ca(OH)2 nanocomposites were also evaluated.
The elemental contents of the produced PAAG@Ca(OH)2 nanocomposite were measured by using energy dispersive X-Ray (EDX) spectroscopy and the crystal structure of the PAAG@Ca(OH)2 nanocomposites was studied using X-Ray diffraction (XRD) measurements. The chemical state of the elements in the PAAG@Ca(OH)2 nanocomposites was determined using X-Ray photoelectron spectroscopy (XPS) measurements.
The effect of PAAG@Ca(OH)2 and AC33 on the mural was assessed using chromatic aberration measurements, while the pore size distribution and porosity were measured using a mercury porosimeter. The "Scotch tape test" (STT) was also used to assess the PAAG@Ca(OH)2 bonding strength.
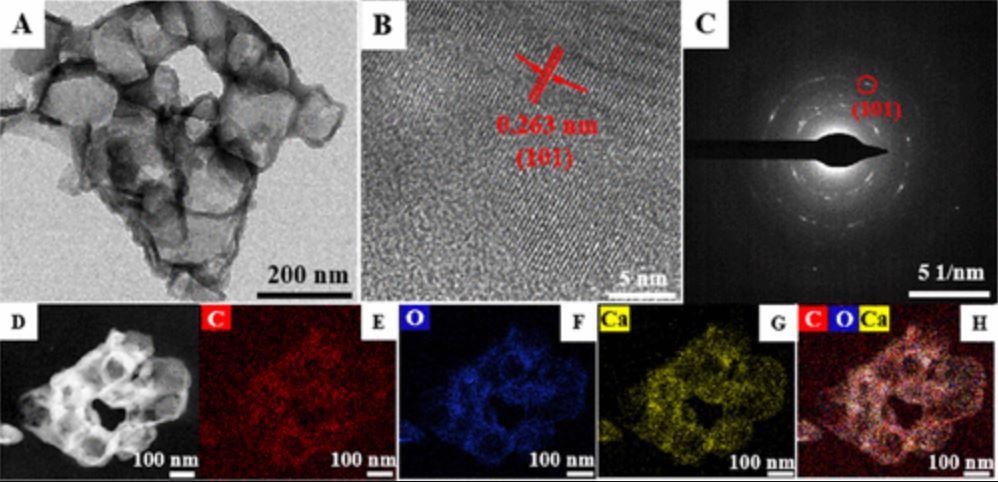
TEM image (A), HRTEM image (B), SAED pattern (C), HAADF–STEM image (D), and elemental mapping images (E–H) of PAAG@Ca(OH)2. © Gu, W., et al (2022)
Research Findings
According to the simulated tests, the nanocomposite PAAG@Ca(OH)2 had higher porosity, suitable hydrophilicity, stronger adsorption, and superior permeability than the commercial AC33 sample.
In the XPS survey spectra of PAAG@Ca(OH)2, three elements - C, O, and Ca - were found. PAAG@Ca(OH)2 had ΔE values less than 5, whereas AC33 had ΔE values more than 5. After being reinforced with PAAG@Ca(OH)2, the hue of simulated samples was not altered much. After reinforcement with AC33, the porosity decreased to 53.2182%, whereas after reinforcement with PAAG@Ca(OH)2, the porosity increased to 54.10%.
The weight loss of the untreated sample, which was not covered with any reinforcing substance, was 3.21 ± 0.5 mg cm-2. Of the AC33-consolidated simulated samples, it was reported to be 1.54 ± 0.5 mg cm-2. The weight loss for the simulated samples consolidated with the PAAG@Ca(OH)2 nanohybrids, on the other hand, was 0.71 ± 0.3 mg cm-2.
PAAG@Ca(OH)2 had a lower moisture exchange effect on the mural, which could be employed to reinforce the pigment layer. The Raman peaks of the AC33 were around 1457 and 1683 per centimeter for the as-prepared AC33 consolidated mural sample, and the intensity appeared from 0 to 200 micrometer and dropped from 200 to 350 micrometer.
Conclusions
In conclusion, this study elucidated the potential of PAAG@Ca(OH)2 nanocomposite as a reinforcement material for mural conservation. The PAAG@Ca(OH)2 manufactured by the researchers could easily capture and store CO2 in the ambient atmosphere due to graphene's huge specific surface area. In addition, simulated experiments showed that the as-prepared PAAG@Ca(OH)2 nanocomposite exhibited strong adsorption, adequate hydrophilicity, and great adherence to mural pigments, making it a potential option for mural consolidation.
The authors emphasized that the PAAG@Ca(OH)2 nanocomposite outperformed the AC33 in terms of conservation efficiency, demonstrating an important application of graphene as a protective material for mural reinforcement.
Reference
Gu, W., Wei, Y., Liu, B., et al. (2022) Polyacrylic Acid-Functionalized Graphene@Ca(OH)2 Nanocomposites for Mural Protection. ACS Omega https://pubs.acs.org/doi/10.1021/acsomega.2c01364.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.