In a recent article published in the journal Scientific Reports, authors reported the synthesis of 3 to 9 nanometer-sized, highly stable, curcumin-functionalized copper nanoparticles (CuC) via chemical reduction. Furthermore, they developed cost-effective CuC paper strips to detect sodium ion (Na+) concentration in urine and blood serum.

Study: Development of a paper printed colorimetric sensor based on Cu-Curcumin nanoparticles for evolving point-of-care clinical diagnosis of sodium. Image Credit: Sadovnikova Olga/Shutterstock.com
Cost-effective Detection of Na+
Abnormal Na+ levels in the human body can lead to cardiac issues and other health problems. The timely detection of Na+ levels in humans can help early diagnosis of hypertension or cardiovascular diseases. Hence the development of Na+ sensors is of high clinical relevance.
Although Na+ concentration detection methods like ion-selective electrodes and ion chromatography give accurate results, their high cost, complex technicality, and high sample requirement limit their regular usage. To this end, paper-based dipsticks are cost-effective, user-friendly alternatives that can give rapid results. However, interfering ions of potassium (K+), magnesium (Mg2+), and zinc (Zn2+) impede the accuracy of Na+ in colorimetric sensing due to their indistinguishable nature.
The unique structural and optical characteristics of metal nanostructures enabled their usage in biomedical applications. In this context, copper nanoparticles (Cu NPs) are ideal candidates for biomedical applications in biosensors, bioimaging, and drug delivery. However, air-sensitive Cu NPs undergo surface oxidation due to the relative stability of copper oxide in the atmosphere. Antioxidant ligands or surfactants are added to prevent this, paving the way for surface-modified Cu NPs.
Application of CuC for Detection of Na+
In the present study, the authors functionalized Cu NP surface with curcumin (a pigment extracted from turmeric) via chemical reduction to protect the Cu NPs from surface oxidation and agglomeration. Further studies on the synthesized CuCs demonstrated high selectivity in sensing and quantifying Na+. Additionally, the authors fabricated sodium sensor strips to detect Na+ visually in the physiological range.
Ultraviolet-visible (UV-Vis) spectroscopy, X-ray diffraction technique (XRD), high-resolution transmission electron microscope (HRTEM), energy-dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectra (FT-IR), infrared (IR) spectroscopy, and dynamic light scattering (DLS) were employed to analyze the physical properties and working mechanism of synthesized CuCs.
Research Findings
The XRD results showed three two theta (2θ) peaks at 43 degrees, 50.32 degrees, and 74.10 degrees aligning with the cubic Cu standard spectrum. FT-IR results of synthesized CuCs and their comparison with the FT-IR spectrum of curcumin showed the attachment of curcumin on the Cu NP’s surface. The spectrum showed the curcumin characteristic peak at 3508-centimeter inverse, attributed to the hydroxyl stretching vibration.
The IR spectra of the synthesized CuCs showed that the aromatic CH vibration peaks at 607 and 633-centimeter inverse in pure curcumin spectra shifted to 602 and 676-centimeter inverse in the synthesized CuC. The disappearance of the peak at 804-centimeter inverse in CuC (exists in pure curcumin) depicted the interaction between Cu NPs and curcumin. The appearance of a peak at 2350-centimeter inverse in CuC (which does not exist in pure curcumin) indicated the formation of the curcumin-Cu complex. Further, the EXD analysis revealed the peak corresponding to Cu, proving the formation of Cu NPs.
TEM images of CuC colloidal samples showed that the cage-like structure of curcumin embeds the Cu NPs clusters. These images also revealed that a group of crystalline Cu NPs with an approximate size of six nanometers combined to form a 100-nanometer diameter cluster. Finally, the HRTEM showed the single-lined curcumin functionalized Cu NPs, where the curcumin layer around Cu NPs prevented their surface oxidation.
The UV-Vis spectra of CuC showed that the characteristic surface plasmon resonance (SPR) intense broad peak of Cu at 803 nanometers shifted to a higher wavelength region due to the bulkiness of CuC.
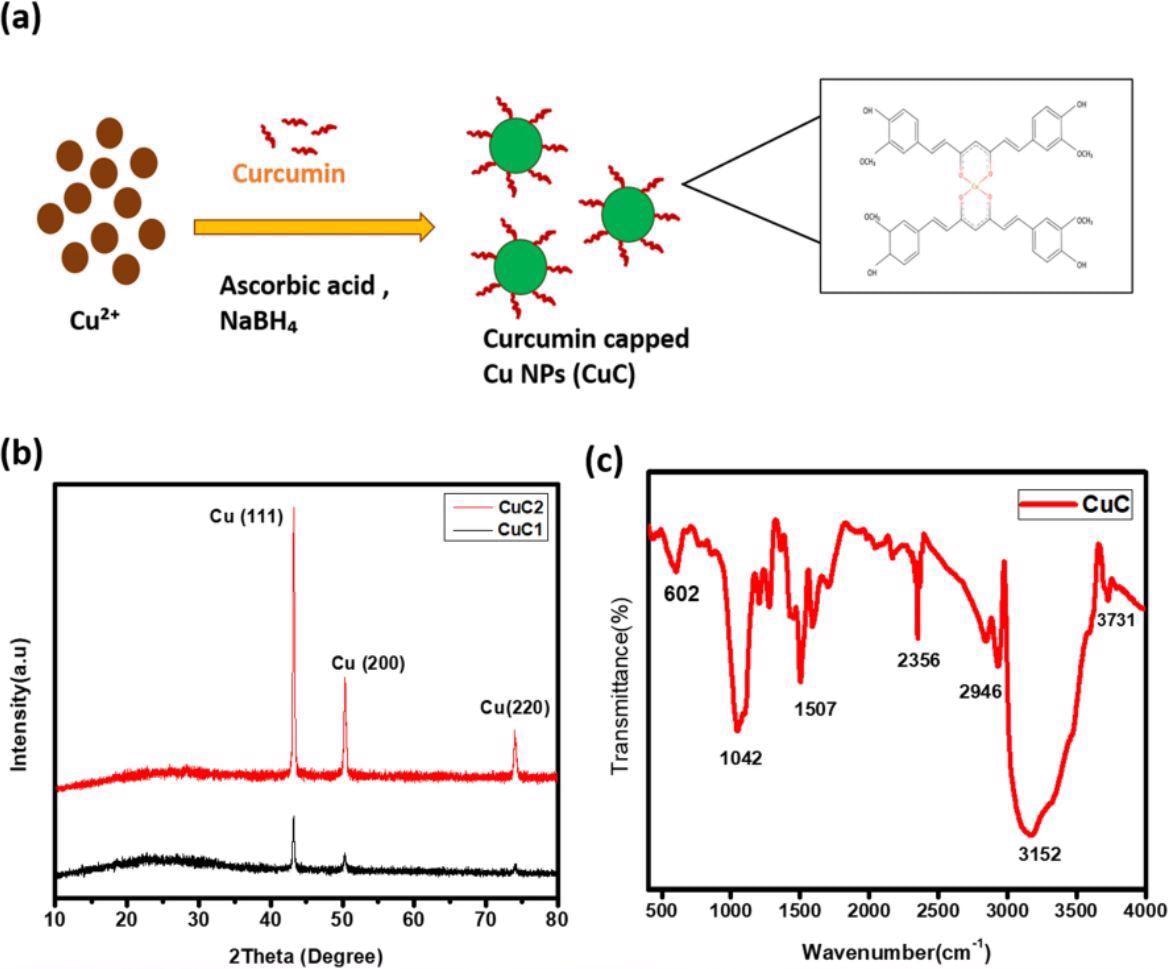
(a) The schematic illustration of formation of curcumin capped Cu NPs (CuC). (b) XRD pattern of curcumin assisted Cu NPs. (c) FTIR spectra of the sample.
Mechanism of Na+ Detection
CuC in Na+ ion solution forms a complex and DSL studies reveal that with an increase in Na+ concentration, the cluster growth increases showed by the exponentially increased peak at 550 nanometers.
The high Na+ concentration induces a positive charge around CuC which inhibits the cluster growth. This is indicated by the appearance of a peak at 2350-centimeter inverse in the FT-IR spectra. Also, the complex with high Na+ concentration produces a deep brown color, which gradually lightens, indicating the sensor’s highest detection limit.
The Na+ selectivity of the calorimetric assay was studied by recording absorbance spectra of CuC containing different metal ion solutions. The results confirmed the selective response of Na+ and no interference of other metals.
Conclusion
Fluctuations in blood and urine sodium content indicate the underlying diseases caused by a disturbance in the body's sodium homeostasis. In these situations, constant monitoring of Na+ concentration is beneficial. Using curcumin-capped Cu NPs, the authors of the present study fabricated a strip-based low-cost colorimetric sensing technique.
The linear absorption response shows the sensor's selectivity to Na+ concentration. The sensor is a shade-card calibrated for known Na+ concentrations and can be used to estimate Na+ concentration in test samples. The high sensitivity and selectivity for the cation in sensor strips help in the facile detection of sodium fluctuations in blood and urine samples.
Reference
Chandran, N., Janardhanan, P., Bayal, M. et al. (2022) Development of a paper printed colorimetric sensor based on Cu-Curcumin nanoparticles for evolving point-of-care clinical diagnosis of sodium. Sci Rep 12, 6247. https://www.nature.com/articles/s41598-022-09852-z
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.