Reviewed by Alex SmithApr 25 2022
Due to their high porosity, vast surface area and ample inner void space, hollow structured nanomaterials have huge potential in the sectors of adsorption, catalysis, energy storage and medication delivery/controlled release.
 Hollow structures with diverse morphologies and adsorption process intensification. Image Credit: Qun Yu.
Hollow structures with diverse morphologies and adsorption process intensification. Image Credit: Qun Yu.
Nevertheless, in a few unusual applications involving big species, the full hollow shell of the hollow nanoparticles works primarily as an obstacle, limiting mass transmission and resulting in inaccessible blank spaces.
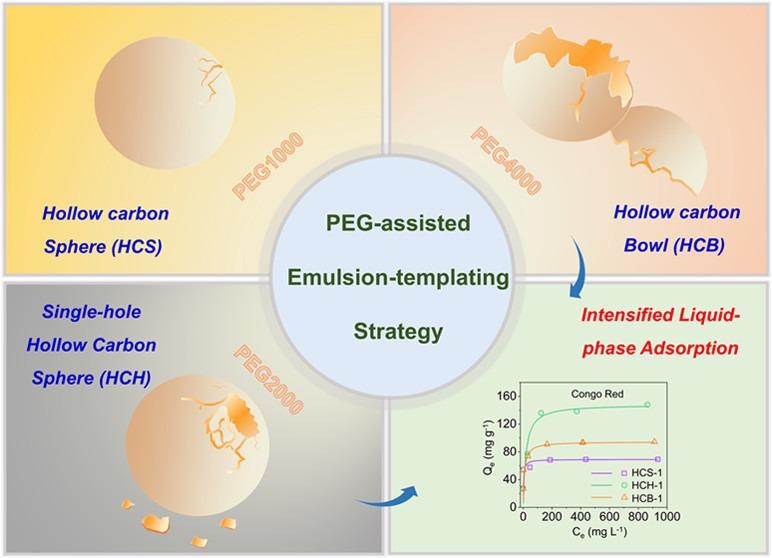
Professor Guanghui Wang of the Chinese Academy of Sciences’ (CAS) Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) has created a new strategy to synthesize single-hole hollow carbon nanospheres (HCH), which has effects on the process expansion of liquid-phase adsorption toward large molecules, in collaborative efforts with Professor Yihan Zhu of Zhejiang University of Technology.
Their findings were published on April 4th, 2022, in Chemistry of Materials.
HCH has the integrated advantages of a hollow cavity and an opening hole in the shell.
In fact, it is challenging to create just a single hole in the shell of hollow structured nanomaterials.
Qun Yu, Doctoral Candidate, Qingdao Institute of Bioenergy and Bioprocess Technology
To make the HCH, the researchers used a poly (ethylene glycol) (PEG)-assisted emulsion-templating technique. The technique occurs in two steps: hydrothermal and pyrolysis. The PEG molecular mass was varied from PEG-1000 to PEG-2000 to PEG-4000 throughout hydrothermal synthesis, and after pyrolysis, hollow carbon structures with a closed shell (HCS), a single hole in the shell (HCH), and a bowl-like shell (HCB) were created.
They observed that the PEG molecules in the emulsion system may be employed as a reverse demulsifier (primarily related to the PEG molecular weight) to cause hollow structures, with or without an opening in the shell, to form. Furthermore, the hollow constructions’ widths and hole sizes may be customized.
According to the researcher's recent study, void-confinement effects caused by void space can influence the hydrodynamics of intermediates and, as a result, the catalytic selectivity in hydrogenation reactions.
The adsorption capabilities and kinetics of HCS/HCH/HCB towards adsorbates of various molecular sizes were investigated. The opening-hole hollow structures, according to the results, can boost the mass transfer of big molecules (e.g., CR) to raise the adsorption rate while also inducing a void-confinement action to improve the adsorption capability.
Our discovery in this adsorption study points to a new direction to reveal void-confinement effects.
Qun Yu, Doctoral Candidate, Qingdao Institute of Bioenergy and Bioprocess Technology
Professor Guanghui Wang comments, “We also envision that such PEG-assisted strategy can stimulate further research in material synthesis, and the HCH can be used not only for fundamental study but also for practical application in adsorption, catalysis, and controlled release.”
The National Natural Science Foundation of China, QIBEBT, and the DNL Cooperation Fund, along with the Zhejiang Provincial Natural Science Foundation of China, supported the study.
Journal Reference:
Yu, Q., et al. (2022) Single-Hole Hollow Carbon Nanospheres via a Poly(ethylene glycol)-Assisted Emulsion-Templating Strategy for Intensified Liquid-Phase Adsorption. Chemistry of Materials. doi.org/10.1021/acs.chemmater.1c04419.