A paper recently published in the journal Advanced Science reviewed the strategy of producing gold nanoparticles (GNPs) through cellular biomineralization in mammalian cells as an alternative to prefabricated GNPs.
Study: Prospecting Cellular Gold Nanoparticle Biomineralization as a Viable Alternative to Prefabricated Gold Nanoparticles. Image Credit: Kateryna Kon/Shutterstock.com
Significance of Cellular Biosynthesis of GNPs
GNPs have demonstrated significant potential in an extensive range of biomedical applications. However, no injectable GNP formulations are clinically approved of, preventing their use in real-world applications. Conversely, gold (Au) salts were used for decades in clinics for rheumatoid arthritis treatment. These treatments with Au salts provided evidence of GNP formation in treated patients in the form of chrysiasis.
Several recent studies evaluating GNP formation in murine models and human cells indicated that the application of Au ions for the in situ theranostic GNP formation could significantly improve the delivery of GNPs in dense biological tissues, GNP formation specificity within cancer cells, and intracellular Au uptake efficiency. These attributes, combined with the safe clinical application of Au salts, can potentially make the in situ GNP formation process in mammalian cells an effective strategy for clinical translation.
In this review, researchers evaluated the Au salt application strategies that can effectively facilitate in situ GNP biomineralization in mammalian cells, acting as a translatable and viable alternative to the classical nanomedicine delivery approach. Researchers also assessed the feasibility of using this new approach for GNP synthesis in biomedical applications.
What is Biomineralization?
Biomineralization is referred to as biotic processes that facilitate the formation of mineral compounds without requiring high-temperature and high-pressure conditions, which are typically associated with inorganic mineralization. Biomineralization occurs extensively in every taxonomic kingdom.
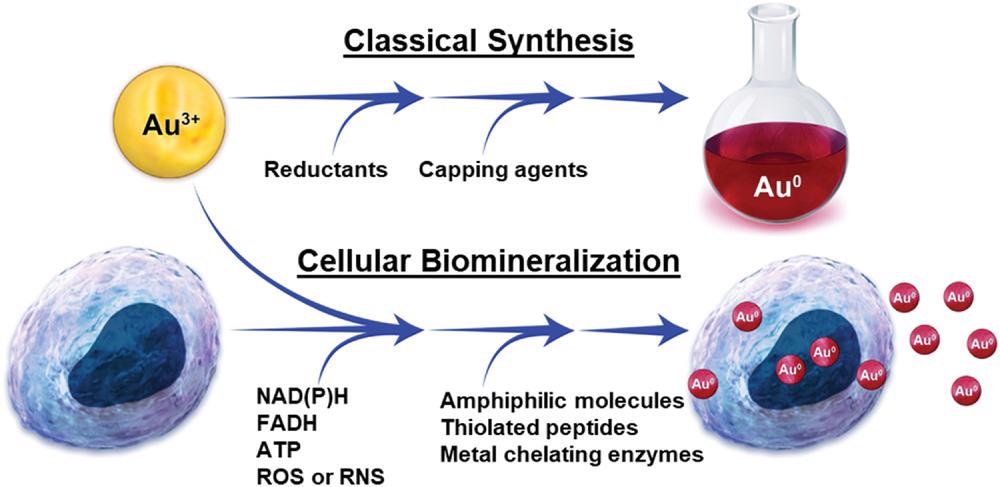
Comparison of a benchtop gold nanoparticle synthesis and gold nanoparticle synthesis through cellular biomineralization. © Schwartz-Duval, A. S., Sokolov, K. V. (2022)
GNP Biomineralization in Mammalian Cells
The synthesis of aqueous GNPs requires a minimum ionic Au concentration with capping and reducing agents. In biological environments such as tissues and cells, these capping and reducing agents are present in the form of biomolecules with capping and reducing properties, except the ionic Au precursor. However, the oxidoreductive cellular redox potential can vary significantly depending on the cell environment and phenotype.
Metabolic biomolecules such as reactive oxygen and nitrogen species (ROS) and (RNS) and adenosine triphosphate (ATP) primarily determine the cellular redox potential, while the amphiphilic molecules that can form metal chelators and micelles or liposomes can act as capping agents during GNP biomineralization. Additionally, the pH and temperature must be restricted to 7.4±0.1 and 37±1 degrees Celsius, respectively, for the potential synthesis of GNPs by reducing ionic Au by mammalian cells.
Studies demonstrated the successful intracellular formation of GNPs through the application of Au salts in the kidney, brain, and cervix cells. Additionally, cellular biomineralization led to the synthesis of fluorescent atomic Au nanoclusters (GNCs), which are less than three nanometers in size and exhibit fluorescent properties. However, GNP biomineralization was more dominant in cancerous cells than in noncancerous cells.
The incubation media conditions significantly influenced the cellular biosynthesis of GNCs/GNPs from ionic Au. Studies indicated that full cell media is necessary for effective fluorescent GNC formation through cellular biomineralization. In contrast, nutrient-free or phosphate-buffered saline (PBS) media is conducive to the formation of plasmonic GNP.
Intracellular GNP biomineralization led to significantly higher intracellular Au content and prominent nuclear localization of Au for every incubation solution compared to prefabricated GNPs over the same period of treatment. However, the biomineralization of GNPs by cells under stress conditions with high Au concentrations resulted in differential nanoparticles compared to normal conditions with low Au concentrations.
Au Biosynthesis Mechanisms
Most of the previous studies were focused on the role of RNS/ROS as drivers of GNP biomineralization through redox potential reactions. However, the RNS/ROS level remains enhanced in cancer pathology, leading to greater GNP biomineralization. Specific biomolecules such as quinone oxidoreductase-like protein (QOH-1) and NADH dehydrogenase (ubiquinine) flavoprotein can facilitate GNP biomineralization.
The inhibition of QOH-1 and NADH using enzyme inhibitors methoxy-1,2-dimethyl-3-[(4-nitrophenoxy) methyl]-1H-indole-4,7-dione (ES93) and rotenone significantly decreased the plasmonic peak intensities of GNPs, which indicated the role of the enzymes in cellular GNP biomineralization.
Glutamate synthesis also influences the GNP biomineralization. For instance compound 968, a glutamate synthesis blocker, substantially reduced the intracellular GNC fluorescence in tumorigenic, spontaneously transformed, and noncancerous cells. GNPs were formed through the direct reaction with sodium glutamate.
Several proteins adhere to GNPs/GNCs formed through cellular biomineralization. For instance, phosphoribosylaminoimidazolecarboxamide formyltransferase (PUR9) and heat shock protein 70 (HSP70) proteins were detected in the extracellular space of biomineralized GNPs formed in the cancerous breast cells and extracted from supernatant space.
Adding Control in the GNP Biosynthesis Process
The specificity of cellular biosynthesis of GNPs can be achieved by adjusting the reaction kinetics in vivo or in vitro and strategies to control and release the delivery of Au ions.
Biomedical Potential of Cells as in situ Nanomedicine and Nanoparticle Factories
Cells can act as biomimetic nanoparticle factories for biomimetic GNP formation. For instance, an extensive range of GNPs can be formed through biomineralization by varying the Au3+ concentration and incubation time. Additionally, in-situ synthesis of GNPs can help in obtaining a better imaging contrast compared to prefabricated GNPs.
For instance, the use of intracellularly formed GNPs in normal human breast epithelial MCF10a as Raman probes effectively resolved the challenges of nuclear delivery and endosomal escape of nanoparticles and provided robust surface-enhanced Raman spectroscopy (SERS) signals from the cytosol and the nucleus in the Raman spectra compared to the prefabricated GNPs.
Intracellular GNP biomineralization has also demonstrated therapeutic potential. For instance, anisotropic Au nanostructures formed by combining GNPs obtained from cellular biomineralization and prefabricated GNPs were more suitable for cancer cell photothermal treatment than the prefabricated polyethylene glycol (PEG)ylated Au nanorods.
Future Outlook of Biomineralized GNPs
Biomineralized GNPs have demonstrated their potential as a feasible alternative to prefabricated GNPs as in situ nanomedicine and biomimetic nanoparticle factories, along with in photothermal therapy and fluorescence image contrast. Thus, the inherently personalized approach can alter the current paradigm in the field of nanomedicine.
However, more extensive mechanistic research is required to understand the mechanisms involved during GNP biosynthesis, determine the time required for biosynthesis in various subcellular locations, and identify the way by which the GNP formation impacts the activity of the involved biomolecules.
Reference
Schwartz-Duval, A. S., Sokolov, K. V. (2022) Prospecting Cellular Gold Nanoparticle Biomineralization as a Viable Alternative to Prefabricated Gold Nanoparticles. Advanced Science. https://onlinelibrary.wiley.com/doi/10.1002/advs.202105957
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.