The composition of protein-nanoparticle complexes can be studied utilizing technologies that enable the investigation of biological molecules' interactions with inorganic compounds. A recent study published in the journal ACS Applied Bio Materials examined the structural characteristics of protein nanoparticle complexes using single-particle cryo-electron microscopy (cryo-EM).
Study: Protein–Nanoparticle Complex Structure Determination by Cryo-Electron Microscopy. Image Credit: Volodymyr Dvornyk/Shutterstock.com
What are Protein-Nanoparticle Complexes?
The combination of nanoparticles with proteins is of considerable interest owing to the incredible potential of their complexes, which combine the nanoscale features of nanoparticles with the particular structure and functionality of different proteins.
Both natural and artificial biological processes are capable of controlling the production and development of inorganic nanoparticles in terms of size, structure, and functionality.
Particularly valuable are nanoparticle–protein complexes in the developing area of nano-bio sciences such as biomedicine, drug development systems, and biosensors. Nanoparticles with sizes equivalent to biological cells can enter and act inside the cell. Studying the interactions of nanoparticles with various protein complexes is therefore required for such applications.
Limitations of Current Structure Determination Methods
At present, there are very few high-resolution investigations of biomolecules interacting explicitly with a synthetic nanocomposite. The most commonly utilized structure determination techniques, X-ray crystallization and nuclear magnetic resonance (NMR), are generally unsuitable structure determination of biomolecules coupled to inorganic substrates.
Methods such as solid-state NMR and sum-frequency generation (SFG) vibrational spectrometry have been utilized effectively to investigate the interactions of smaller biomineralizing protein complexes with inorganic materials.
Despite the fact that these approaches provide extremely valuable data, they are presently confined to smaller proteins, and several supplementary procedures are necessary to evaluate the structural information.
Cryo-Electron Microscopy (cryo-EM): A Novel Structure Analysis Technique
The investigation of the biomolecule-inorganic interface region, as well as the subject of biomaterials, would benefit immensely from functional and better structural analytical techniques. One such method, cryo-electron microscopy (cryo-EM), has transformed molecular biology in past years.
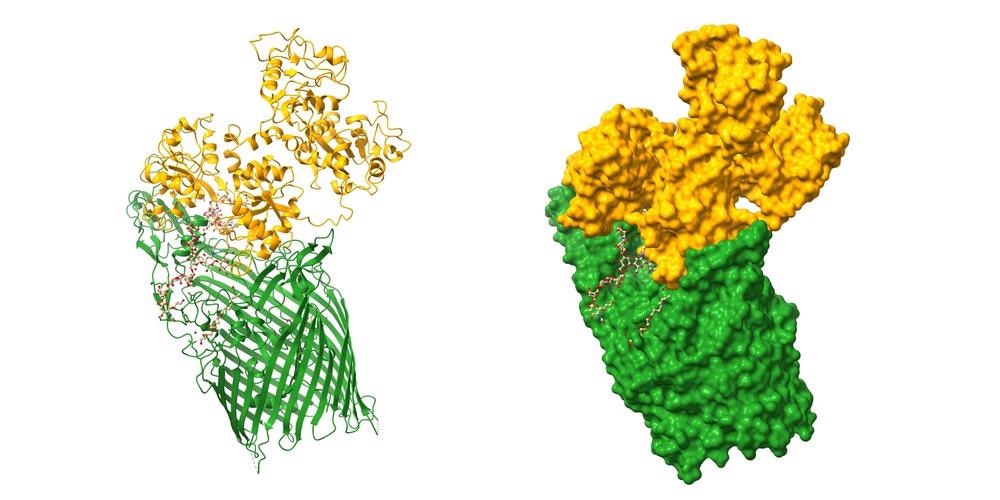
In this study, the researchers wanted to show how single-particle cryo-EM approaches may be used to analyze protein nanomaterial compounds. In the study, two protein nanomaterial compounds, GroEL linked with platinum nanoparticles (GroEL-PtNP) and ferritin attached to iron oxide nanoparticles, were used as model materials.
Key Developments of the Study
It was demonstrated that cryo-EM could be utilized to identify high-resolution protein structures and nanoscale materials in the instance of a GroEL and platinum nanoparticle (PtNP) combination. GroEL is a 60 kDa protein that helps several proteins fold and is required for cell survival. GroEL has been found to help create stable PtNPs in addition to its crucial functions in protein folding.
Next, the researchers employed ferritin linked with iron oxide nanoparticles (HuLF-FeNP) to see whether additional protein nanomaterials complexes could be studied using normal cryo-EM methods.
Ferritins are a kind of biomineralizing protein that plays an important role in preserving iron levels inside molecules. Ferritin concentrations in the body are changed in various chronic diseases.
Ferritins have several uses in biotechnology, including directed production of organic semiconductors, neuroimaging, and biomolecule-based water filtration systems, in addition to their vital function in iron homeostasis.
The precision of the cryo-EM was substantially worse in the case of HuLF-FeNPs, and a definite structure and composition could not be determined. While the cryo-EM data did not grant an adequate calibration of the atomic model for HuLF, the iron oxide nanoparticles could still be seen on the HuLF shell's internal surfaces.
Future Perspective on Cryo-EM and its Applications
It can be concluded from the findings that single-particle cryo-EM has shown great promise for studying the structural properties of protein-nanomaterial interactions. However, more advancements will be necessary to produce high-resolution models of the protein-inorganic interfaces.
While the data obtained was used to create a structure using pretty typical techniques, there are various workflow enhancements that might improve the final outcomes. Future research on HuLF-FeNPs and GroEL-PtNPs should concentrate on improving masking processes in order to increase the quality and resolution of 3D data produced from these samples.
It is anticipated that future advancements to cryo-EM data collecting and processing techniques can increase the clarity of the models and provide vital new structure-function details into how organic substances engage with inorganic compounds.
Reference
Sen, S. et al. (2022). Protein–Nanoparticle Complex Structure Determination by Cryo-Electron Microscopy. ACS Applied Bio Materials. Available at: https://pubs.acs.org/doi/10.1021/acsabm.2c00130
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.