By Matt Pullen, B.Sc.Reviewed by Matt Pullen, B.Sc.Jun 8 2022
Yolk-shell nanocrystals are one-of-a-kind materials with intriguing structural features, including a permeable shell, inner space, and moveable yolk. Depending on the materials that are used in producing them, these nanocrystals can be utilized for a wide range of purposes.
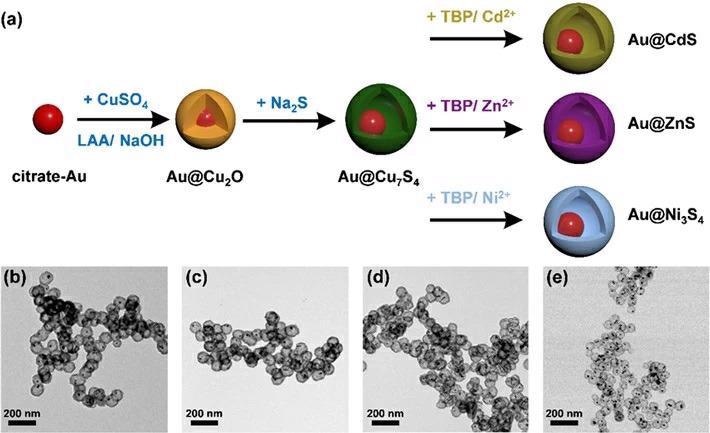 (a) Schematic depiction of the synthetic procedure for Au@Cu7S4, Au@CdS, Au@ZnS, and Au@Ni3S4. (b-e) shows the corresponding TEM images. The synthesis of yolk-shell nanostructures involves sulfidation on an Au@Cu2O core-shell nanocrystal template to convert the shell composition to various metal sulfides. Image Credit: Tokyo Institute of Technology
(a) Schematic depiction of the synthetic procedure for Au@Cu7S4, Au@CdS, Au@ZnS, and Au@Ni3S4. (b-e) shows the corresponding TEM images. The synthesis of yolk-shell nanostructures involves sulfidation on an Au@Cu2O core-shell nanocrystal template to convert the shell composition to various metal sulfides. Image Credit: Tokyo Institute of Technology
Egg yolk-shell nanocrystals, for example, can be used to build a dependable photovoltaic device if the inner surface of their shells is reflective. A movable core can be used as a stirrer, allowing solutions kept within the shell to be mixed.
The yolk-shell structure’s fascinating properties (an outcome of electronic interactions and charge transfer between the structure’s surfaces) end up making these nanocrystals ideal for photocatalysis applications.
The inner and outer surfaces of the shell have plenty of active sites for reactions. Researchers all over the world are interested in yolk-shell nanocrystals, which is understandable.
A global research team led by Associate Professor Tso-Fu Mark Chang and Assistant Professor Chun-Yi Chen of the Tokyo Institute of Technology (Tokyo Tech), and Professor Yung-Jung Hsu of the National Yang Ming Chiao Tung University in Taiwan, has devised several yolk-shell structures containing a metallic gold (Au) yolk with many semiconductor shells.
Due to their interesting features, mainly because of their Au cores, such structures have grown in popularity across the world.
Yolk-shell nanocrystals comprising of a metal yolk and semiconductor shells are particularly interesting because they can be geared to mass transport-related utilizations, for example, photocatalysis.
Chun-Yi Chen, Assistant Professor, Tokyo Institute of Technology
The researchers used a sequential ion-exchange technique to create the nanocrystals. A delicate sulfidation on an Au@Cu2O core-shell nanocrystal template (where Au contributes to the core and Cu2O to the shell formation) is followed by a kinetically controlled cation exchange reaction that allows the conversion of the shell composition (i.e., Cu2O) into various metal sulfides, which are semiconductors.
Four exemplary yolk-shell nanocrystal samples were manufactured for examination, including Au@Cu7S4, Au@CdS, Au@ZnS, and Au@Ni3S4.
X-ray photoelectron spectroscopy (XPS) and steady-state photoluminescence (PL) spectroscopy were used to assess the effectiveness of these yolk-shell structures as photocatalysts.
The researchers discovered that the metal cores and semiconducting shells of the nanocrystals exhibit favorable electronic interactions for photocatalysis applications using XPS.
The nanostructures had a high PL intensity, showing significant photocatalytic activity, signifying that they were highly capable of absorbing light and producing electron-hole charge carriers, according to time-resolved PL spectroscopy.
Explaining the scenario where the novel yolk-shell photocatalysts could be used, Prof. Chen commented, “In a real-world scenario, the reactions facilitated by separated photoexcited electrons and holes play a role in environmental purification, by producing reactive oxygen species.”
These photoexcited electrons and holes may speed up a variety of processes, making yolk-shell nanocrystals useful in a wide range of applications, including environmental cleaning, hydrogen synthesis, and carbon dioxide reduction.
Journal Reference:
Wu, J.-Y, et al. (2022) Electronic Interactions and Charge-Transfer Dynamics for a Series of Yolk–Shell Nanocrystals: Implications for Photocatalysis. ACS Applied Nano Materials. doi:10.1021/acsanm.2c01529