Matter exhibiting chirality in biological systems shows a unique optical response under polarized light. However, morphological control and the consequent optical response of 3-dimensional (3D) chiral nanoparticles (NPs) is challenging.
Study: Adenine oligomer directed synthesis of chiral gold nanoparticles. Image Credit: posmguys/Shutterstock.com
In an article published in Nature Communications, researchers demonstrated the synthesis of chiral gold (Au) NPs utilizing a chiral shape modifier single-stranded (ss) oligonucleotide.
The homo-oligonucleotide was composed of adenine nucleobase and showed chirality evolution with a dissymmetric factor of 0.04 at visible wavelength, while other nucleobases did not show any chirality development. The synthesized Au NPs showed a counterclockwise rotation of the generated chiral arms, with 200 nanometers edge length.
Density functional theory (DFT) simulations and molecular dynamic (MD) studies revealed that adenine showed high enantioselective interactions with Au (321)R/S facet in terms of its affinity and binding orientation which attributed to intra-strand hydrogen (H2) bonding formed between nucleobases. The variation in sequence programming of cytosine and adenine-based oligomers resulted in chiral Au NP’s morphological and optical change.
Chiral NPs
The non-superimposable geometrical orientation of matter with its mirror image is termed chirality and exists in biological systems. In the presence of circularly polarized light, the chiral matter shows a unique optical response, which inspired the development of artificial chiral nanostructures by utilizing plasmonic materials. These artificial plasmonic chiral nanostructures leverage the electromagnetic fields on their surface that can amplify the sensitivity in enantioselective catalytic reactions and chirality detection.
Previous reports on biosensors based on the localized surface plasmon resonance (LSPR) phenomena utilized the chiral plasmonic nanostructures. These nanostructures enhanced the sensitivity of the materials towards surrounding media like refractive index, and the changes were detected using chiroptical spectroscopic methods. Moreover, enhancement in the optical chirality around chiral plasmonic nanostructures showed chiral discrimination with higher sensitivity.
Imparting helicity to plasmonic nanomaterials via chiral biomolecules incorporated during the preparation can develop intrinsic chirality in them. Moreover, the direct transfer of molecular chirality by using chiral starting material in the preparation of inorganic nanomaterials is a facile method to synthesize chiral nanomaterials and diversify their applications.
The four components of DNA oligomers, namely adenine, cytosine, thymine, and guanine, have nano-structuring capabilities. The facile programmability of sequence, specific hybridization properties owing to complementary sequences, and surface interactions with inorganic materials are a few characteristics of DNA that confirm their applicability as basic units for nanomaterials.
Based on previously reported simulation and experimental studies, the affinity trend of DNA nucleobases towards the Au surface is in the order of adenine>cytosine>guanine>thymine. These surface interactions based on nucleobases induced morphology control at a single NP level in Au-based monometallic and bimetallic systems. However, the surface interaction studies based on nucleobases remain unclear concerning chiral induction and morphology.
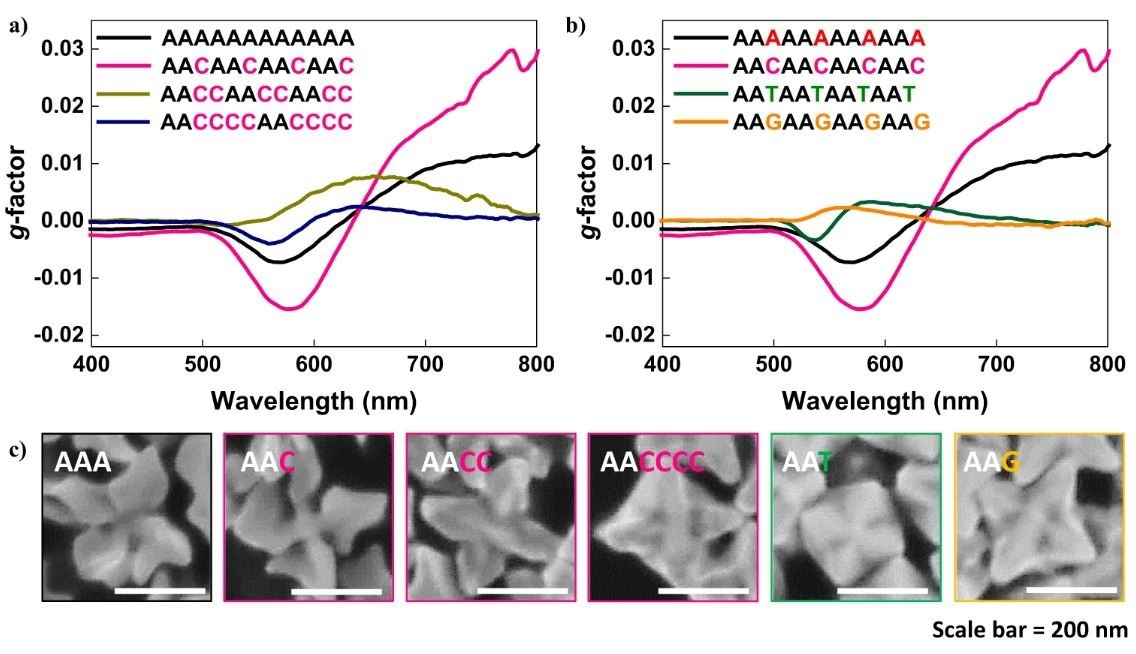
Oligomer sequence-programmed chirality evolution. a The chiroptic response of chiral gold nanoparticles synthesized with a 12-mer oligomer with consecutive Cytosine spacer length control from 0, 1, 2, and 4. b The chiroptic response of chiral gold nanoparticles synthesized with a 12-mer oligomer with spacer nucleobase control while maintaining consecutive Adenine length constant at 2. c High-magnification SEM image of sequence-programmed gold nanoparticle synthesis.
Adenine-based Oligomer Directed Synthesis of Chiral Au NPs
In the present study, the researchers synthesized Au NPs and demonstrated their generation of adenine-specific chirality. The chirality evolution was illustrated by incorporating adenine-oligomer during the NP synthesis, with about 0.04 dissymmetric factor at the visible region wavelength. Apart from adenine, the other nucleobases with deoxyribose sugar molecules and chiral centers did not show chirality-inducing capability.
Scanning electron microscopy (SEM) and circular dichroism (CD) spectrometers demonstrated the variation in chirality evolution. The chirality in synthesized NPs was quantified using a dissymmetric factor, calculated from CD and extinction values. Furthermore, the MD and DFT calculations helped interpret the generation of adenine-specific chirality.
The MD simulations revealed that an intra-base hydrogen bond formed in adenine induced the enantioselective interaction and controlled the relative orientation of the oligomer on the high-index Au surface. Sequence tuning of ssDNA oligomers resulted in variation in the NP morphology and induced their chiroptic response.
Conclusion
In summary, adenine-based ssDNA was used to synthesize chiral NPs. This generation of chirality in NPs affected the chiral response, and their dissymmetric factor reached 0.04 at 650 nanometers. Moreover, four chiral arms generated in Au NPs due to the interactions between A50 oligomer and Au surface showed an anti-clockwise rotation of the chiral motif, with each arm protruding from the center point in each plane, representing octopod-like boundaries.
The simulation studies revealed the vital role of nucleobase chirality that determined their orientation on the Au surface and the difference in adsorption energy. The high enantioselectivity in adenine resulted in a prominent surface orientation difference on S and R surfaces, which showed an energy difference of 0.016 electronvolts per molecule.
Based on the specific interaction capability of nucleobases, an adenine-based oligomer sequence could induce chirality. The consecutive adenine length and type of spacer greatly influenced the generation of chirality in NPs and led to the chiroptic response. These results provided an insight into chiral nanomaterials synthesis with tunable geometrical and optical properties.
Reference
Cho, NH., Kim, YB., Lee, YY., Im, SW., Kim, RM., Kim, JW et al. (2022) Adenine oligomer directed synthesis of chiral gold nanoparticles. Nature Communications. https://www.nature.com/articles/s41467-022-31513-y
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.