The creation of a flexible microfluidic nanoplasmonic detector capable of refreshable and transportable fingerprint identification utilizing high surface-enhanced Raman scattering (SERS) activity is the subject of a recent study published in the journal npj flexible electronics.
In contrast to traditional wearable SERS platforms, which risk having mixed effects from recent and old sweat, the microfluidic SERS device permits sweat handling in a regulated and high temporal-resolution manner, allowing for refreshable SERS evaluation.
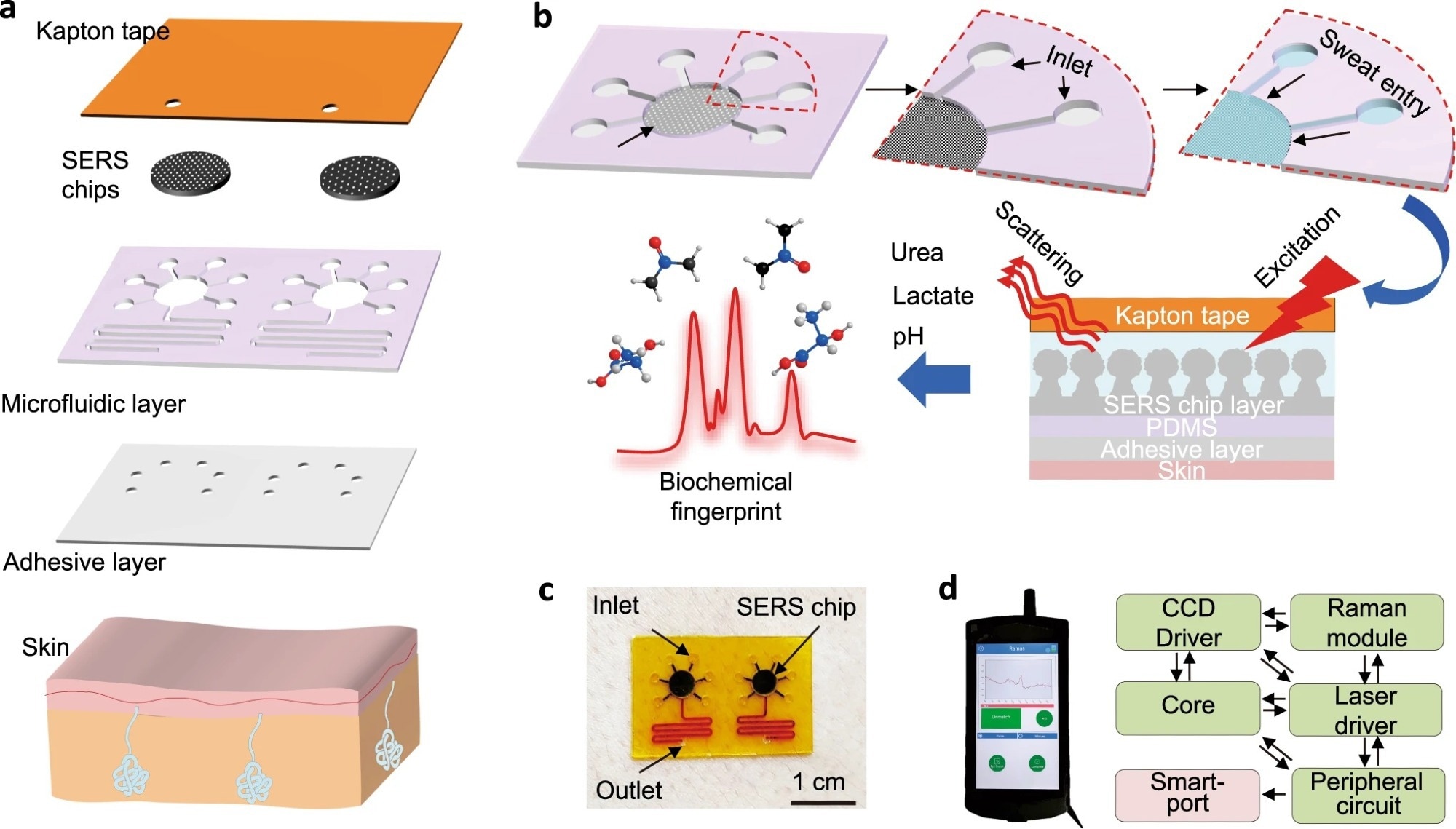
Figure 1: Design of epidermal sweat sensor based on wearable SERS microfluidic platform. a Stacked view showing each layer of the integrated device. b Schematic of all-in-one biofluid flowing, store, and SERS analysis based on a microfluidic plasmonic device. c Actual image of an as-prepared microfluidic SERS sensor attached to the skin. d System-level block diagram showing the internal functional modules of the portable Raman analyzer.
Wearable Sweat Sensors: Overview and Significance
Flexible, intelligent devices have transformed the perception, methodology, and strategies of human-machine interaction and healthcare applications. Wearable sweat sensors that allow the recognition of molecular-level traces in epidermally accessible sweat are considered crucial biomedical detection devices.
By integrating molecular identification methods, micro-nano electronics, interconnected hardware/software platforms, and different analytical approaches, significant development can produce these wearable sweat detectors. The wearable sweat detectors can now provide visual sensing access through color/absorbance and fluorescent depth measurements.
Electrochemical approaches for fabricating wearable sweat sensors offer great specificity and sensitivity, with electrodes that can be built in many forms and sizes.
However, each of these strategies has several advantages and disadvantages. As a result, ongoing innovation in developing novel signal reading methods is required to provide better possibilities for creating wearable sweat detectors.
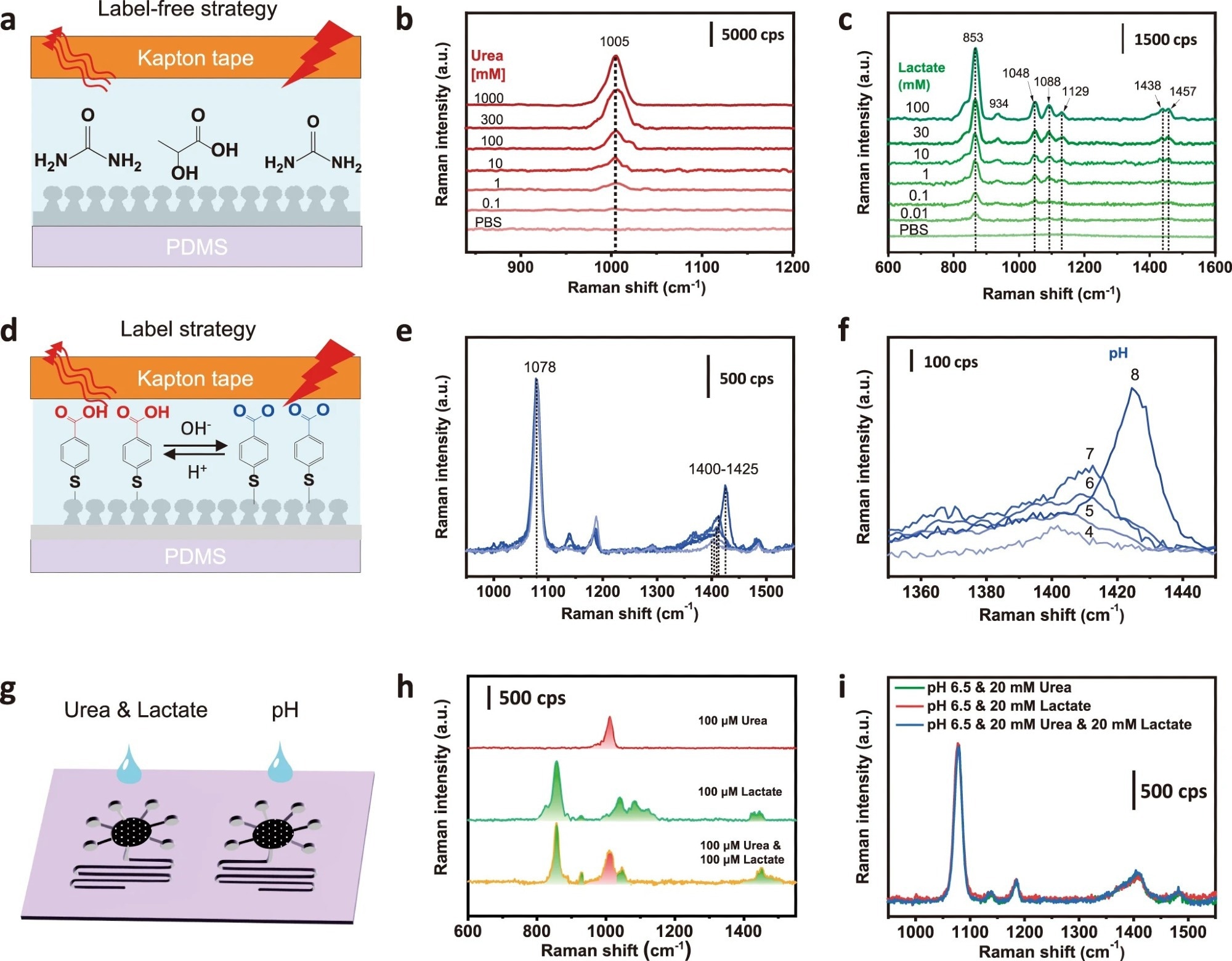
Figure 2. SERS sensing of targeted analytes in standardized solutions. a Schematic and SERS spectra of b urea and c lactate in a label-free manner. d The mechanism of pH SERS sensing showing the probe molecule of 4-MBA labeled on the Ag surface, which can be protonated at an acidic pH and deprotonated at a basic pH. e SERS spectra and f the enlarged view of the Raman peak at 1400-1425 cm−1 responded to different pH levels after normalizing the peak intensity at 1078 cm−1. g Schematic of simultaneous and multiple SERS analysis of sweat targets based on one microfluidic chip. h Compatibility study of simultaneous SERS detection of urea and lactate. i Interference study of pH SERS sensing.
SERS Integrated Flexible Plasmonic Devices
SERS is a widely used analytical method that provides strong Raman signal augmentation by targeted plasmon-enhanced stimulation and dispersion. Flexible plasmonic electronics created by combining SERS with wearable technologies have received a lot of interest for smart biomedical applications.
To date, only a few portable SERS sweat detectors have been documented in the literature. Sweat-permeable SERS substrates enable sweat to drain and populate hotspots in these non-microfluidic sweat detectors. However, these permeable SERS substrates are often physically fragile and prone to epidermal distortion when in contact with the skin.
Another important difficulty with the previously suggested wearable sweat SERS systems is the reading method. The traditional heavy Raman equipment restricts the study of wearable SERS sensors in regulated laboratory conditions, thus limiting their usefulness.
A Novel Microfluidic SERS-based Flexible Sweat Sensor
SERS substrates may be spatially controlled and customized in a flexible manner using microfluidics. Furthermore, dynamical sweat transferring allowed by microfluidics can reduce the blending and carry-over impact of old and fresh sweat.
In this study, the researchers created a flexible microfluidic nanoplasmonic detector in for the reusable and transportable detection of sweat molecular fingerprints.
For transportable identification of sweat biomarkers, a compact and tailored Raman analyzer with a comfortable human-machine interface was deployed.
Key Developments of the Study
Compared to standard non-microfluidic detectors, this microfluidic biodevice enables the adjustable selection of a highly-active SERS substrate, giving high temporal resolution and refreshable sweat SERS analysis.
The portable SERS scanner can interpret sweat fingerprint data of selected biomarkers of urea, lactate, and pH at the cellular scale, boosting the applications of wearable SERS devices for point-of-care testing (POCT).
Adopting a portable Raman scanner also allows Raman signals to be read anywhere, eliminating the need for expensive equipment and conventional laboratory conditions. The plasmonic microfluidic device can thus provide a medical research environment for customized healthcare by allowing for the quick, simple, and portable screening of physiologically linked indicators in sweat.
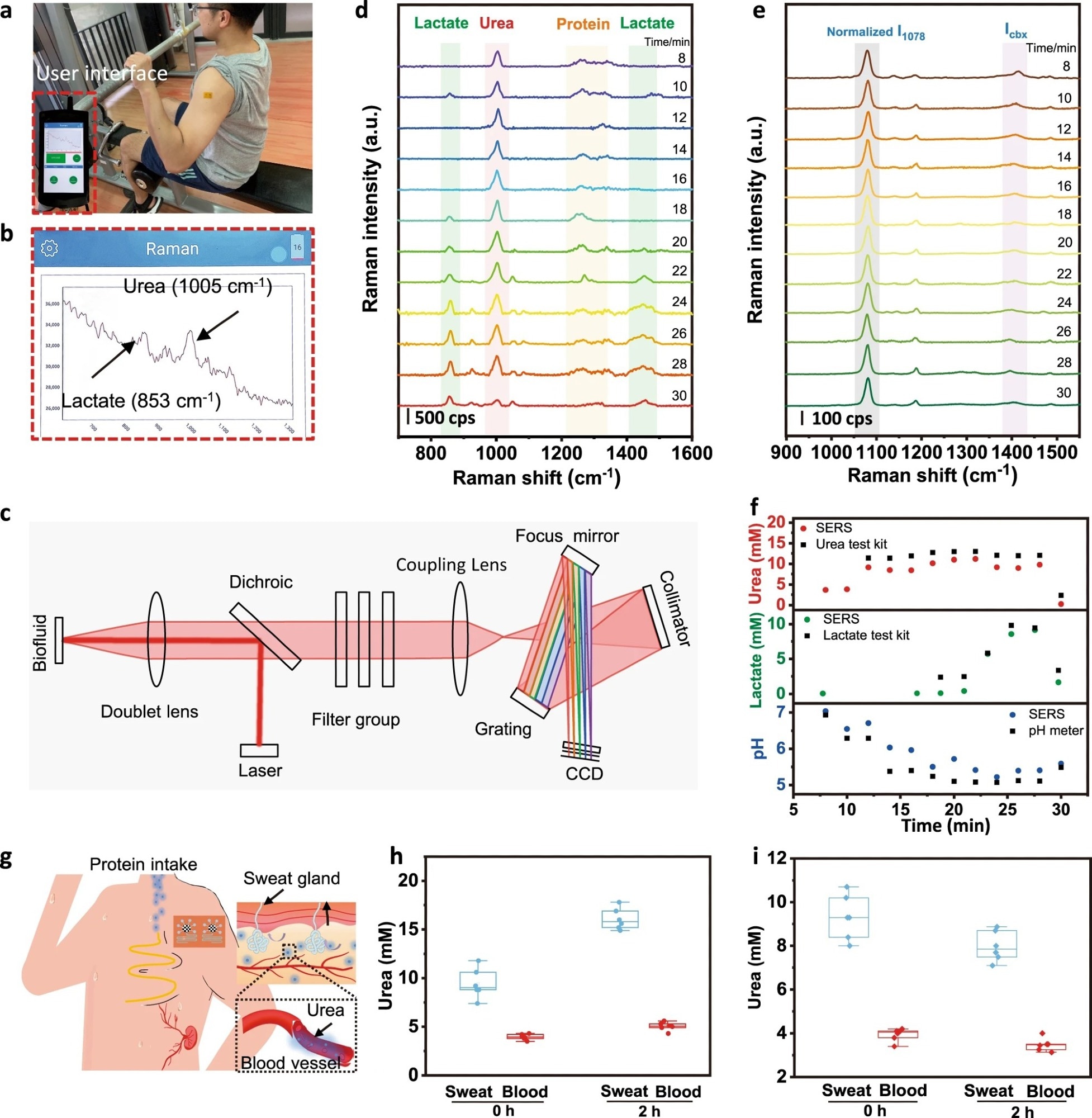
Figure 3. On-body evaluation of the microfluidic sweat SERS chip towards personalized medicine. a Photograph of a volunteer wearing a microfluidic SERS patch during continuous exercise. The inset indicates the portable Raman analyzer. b The user interface and c system-level beam path diagram of the portable Raman analyzer. Discrete Raman spectra of d sweat urea, lactate, and e pH as well as f the corresponding contents calculated from the above calibration curves. The color and black data correspond to measurements done by SERS and by commercial benchmark methods (urea test kit, lactate test kit, and pH meter), respectively. g Schematic showing the metabolic behavior of urea in the human body. Evaluation of the microfluidic SERS device in dietary challenges by comparing the sweat urea and serum urea h with and i without protein intake (n = 6, the points represent raw data; the five lines from bottom to top represent minimum, lower quartile, median, upper quartile and maximum, respectively).
Future Outlook
Despite promising results, some issues concerning the design of portable and wearable SERS biofluid detectors are still prominent, such as possible peak overlap in the case of repeated SERS tests. In this context, creating a highly homogenous substrate resistant to mechanical distortion is required for repeated SERS measurements.
The adaptability of the transportable Raman analyzer in a broader range of analytes should also be investigated by gathering additional experimental data.
Regardless, the researchers have made significant progress in this work in the creation of a reusable sweat biosignal sensor for non-invasive molecular probing. As a result, it is safe to assume that these wearable sensors will reveal innovative opportunities and open up previously unrecognized frontiers in various sectors, including criminal surveillance, medical services, and intelligent healthcare.
Reference
Xuecheng He. et al. (2022). Flexible microfluidic nanoplasmonic sensors for refreshable and portable recognition of sweat biochemical fingerprint. npj flexible electronics. Available at: https://www.nature.com/articles/s41528-022-00192-6
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.