Although biomass-derived carbon quantum dots are sustainable nanomaterials, they have poor optical properties and low photoluminescence quantum yield (PLQY). An article published in the journal Scientific Reports reported the synthesis of nitrogen (N)-doped carbon quantum dots from biomass precursors (glucose and ammonia) with outstanding optical properties and considerable PLQY.
Study: Investigating the effect of N-doping on carbon quantum dots structure, optical properties and metal ion screening. Image Credit: GiroScience/Shutterstock.com
Here, the concentration of ammonia (nitrogen dopant precursor) determined the optical properties of carbon quantum dots. The optimized nitrogen-doped carbon quantum dots exhibited improved fluorescence emission properties with a PLQY of 9.6% and a stable pH range between 2 and 11.
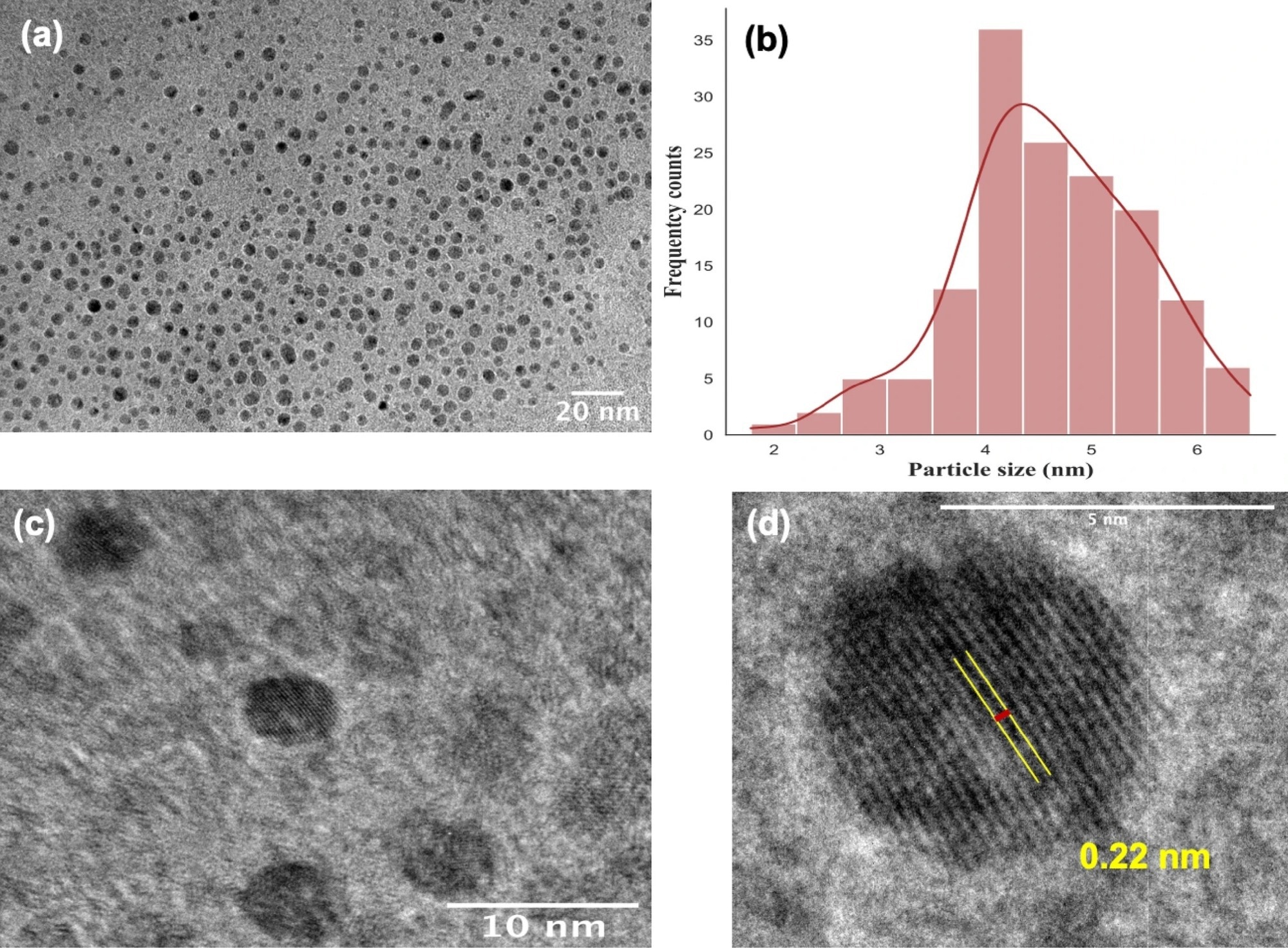
Figure 1. HRTEM images of N-CQDs at different magnification and scale: (a) 20 nm, (c) 10 nm, (b) particle size Gaussian distribution histogram, (d) graphitic core lattice. The N-CQDs have commonly a particle size of 4.60 ± 0.87 nm. © Nguyen, K.G. et al. (2022).
The optimized nitrogen-doped carbon quantum dots served as sensitive nanosensors for quantifying chromium (Cr) (VI) ions. Moreover, the nitrogen-doping process tuned the electronic structure of nitrogen-doped carbon quantum dots, resulting in enhanced optical and nanosensing performance.
Biomass Derived Carbon Quantum Dots
High-performance carbon quantum dots have applications in chemical sensing, bioimaging, nanomedicine, solar cells, optronics, and catalysis. Although synthetic approaches like top-down and bottom-up were proposed to synthesize carbon quantum dots, these processes have drawbacks, including multistep synthesis, high costs, and the use of toxic substances.
Carbon quantum dots are three-dimensional (3D) clusters with a spherical-like structure composed of carbon atoms and tiny amounts of molecules. The inner parts of the 3D clusters are mainly composed of sp3 hybridized carbon atoms and a small portion of sp2 hybridized carbon atoms.
Biomass is an abundant, complex, heterogeneous, biodegradable, and bio-organic substance that may be obtained from diverse sources such as organic domestic garbage, perennial grass, fishery, agricultural residues, poultry, animal husbandry, and forestry. This biomass waste is abundant in carbon content (45–55%) and is considered a renewable source to synthesize nanomaterials via an eco-friendly process, reducing the overall production cost.
Although biomass-derived carbon quantum dots are desirable, the synthetic processes involved are associated with limitations of poor control over particle size, homogeneity, and product quality. These biomass-derived carbon quantum dots come with low PLQY and poor optical properties.
Doping the carbon quantum dots with a heteroatom such as nitrogen (N), phosphorous (P), and sulfur (S) is a common approach to improve their optical properties. However, the improved optical properties via heteroatom doping were only theoretically hypothesized, while its practical achievement is still questionable owing to its high energy consumption and longer synthesis time.
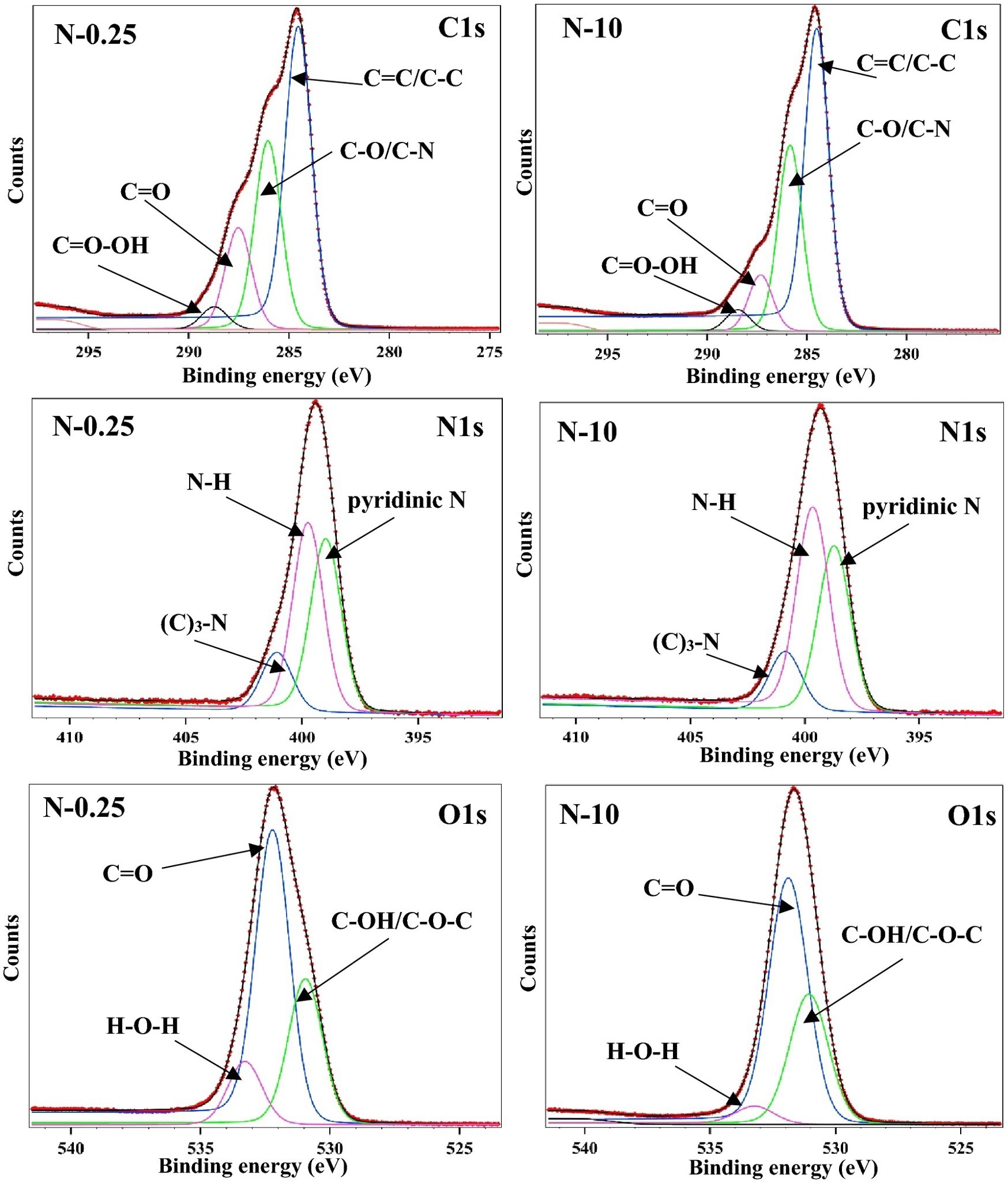
Figure 2. Representative XPS spectra of N-CQDs showing the lowest (N-0.25) and highest (N-10) nitrogen doped samples. The spectra display three typical peaks C1s (285.0 eV), N1s (399.0 eV), and O1s (531.0 eV). The deconvoluted N1s band showed three peaks representing pyridinic N, N–H and amide C–N. © Nguyen, K.G. et al. (2022).
N-Doped Carbon Quantum Dots with Improved Optical Properties
Although various synthetic methods were proposed to synthesize carbon quantum dots from biomass materials, the synthetic routes had poor control over particle size, homogeneity, and quality. Thus, biomass-derived carbon quantum dots exhibited poor optical properties and low PLQY.
In the present work, a water-based continuous hydrothermal flow synthesis (CHFS) was adopted to prepare the N-doped carbon quantum dots from glucose and ammonia. Thus, the current synthetic approach was considered a promising green approach for synthesizing carbon quantum dots.
Surface functionalization and nucleation processes of nanoparticles via CHFS allowed the tailoring of nanoparticles for specific functions. Moreover, the CHFS consumed less time and energy than the other conventional hydrothermal processes in producing a quality product, suggesting that this synthetic strategy toward carbon quantum dots is robust and eco-friendly.
In addition, the continuous hydrothermal process helped control the particle size via nucleation and heteroatom doping without additional post-treatment, suggesting that a current method is a cost-effective approach to synthesizing heteroatom-doped carbon quantum dots. The biomass-derived carbon quantum dots were prepared from readily available and abundant glucose and ammonia as carbon and nitrogen sources, respectively, reducing the overall production cost.
Furthermore, the unresolved concern regarding improved optical properties via heteroatom doping of carbon quantum dots was addressed in the present work. The fabricated N-doped carbon quantum dots were explored for their optical characteristics, and the results revealed excellent optical properties, PLQY of approximately 10%, and pH stability between 2 and 11.
Moreover, the chemical nanosensing of the N-doped carbon quantum dot-based nanosensor helped in quantifying the toxic Cr (VI) ion, which is a carcinogenic, hemotoxic, and genotoxic metal with a limit of detection (LOD) value of 0.95 parts per million and a limit of quantitation (LOQ) value of 3.18 nanometers.
![(a) PLQY and non-radiative rate (GlU?=?g-CQDs), (b) PL lifetime of g-CQDs and N-CQDs. The analysis revealed an increase in both PL lifetime and PLQY upon nitrogen doping and the highest values of lifetime and PLQY were obtained for [N]?=?7.5 M.](https://www.azonano.com/images/news/ImageForNews_39572_16608197737362758.jpg) Figure 3. (a) PLQY and non-radiative rate (GlU = g-CQDs), (b) PL lifetime of g-CQDs and N-CQDs. The analysis revealed an increase in both PL lifetime and PLQY upon nitrogen doping and the highest values of lifetime and PLQY were obtained for [N] ≥ 7.5 M. © Nguyen, K.G. et al. (2022).
Figure 3. (a) PLQY and non-radiative rate (GlU = g-CQDs), (b) PL lifetime of g-CQDs and N-CQDs. The analysis revealed an increase in both PL lifetime and PLQY upon nitrogen doping and the highest values of lifetime and PLQY were obtained for [N] ≥ 7.5 M. © Nguyen, K.G. et al. (2022).
Conclusion
To conclude, N-doped carbon quantum dots were efficiently synthesized from ammonia and biomass precursors (glucose) via the CHFS process. The synthesized N-doped carbon quantum dots showed outstanding optical properties, PLQY of approximately 10%, and pH stability between 2 and 11.
Moreover, the synthesized N-doped carbon quantum dots were tested for their potential as a chemical sensor for quantifying Cr (VI) ions. The results showed LOD and LOQ values of 0.95 parts per million and 3.18 nanometers, respectively.
Furthermore, the fluorescence lifetime studies revealed the quenching mechanism of N-doped carbon quantum dot-based nanosensors, which was an inner filter effect (IFE). Thus, the present work demonstrated a rapid, novel, single-step, and green approach toward synthesizing carbon quantum dots, used for a wide range of applications.
Reference
Nguyen, K.G. et al. (2022). Investigating the Effect of N-Doping on Carbon Quantum Dots Structure, Optical Properties, and Metal Ion Screening. Scientific Reports. https://www.nature.com/articles/s41598-022-16893-x
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.