A multinational team led by the French École Polytechnique, the University of Rostock, and the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) conducted a novel experiment to find out what occurs in the planets’ cores, such as those of Neptune and Uranus. The researchers shot a laser at a basic sheet of PET plastic to observe the action with powerful X-ray flashes.
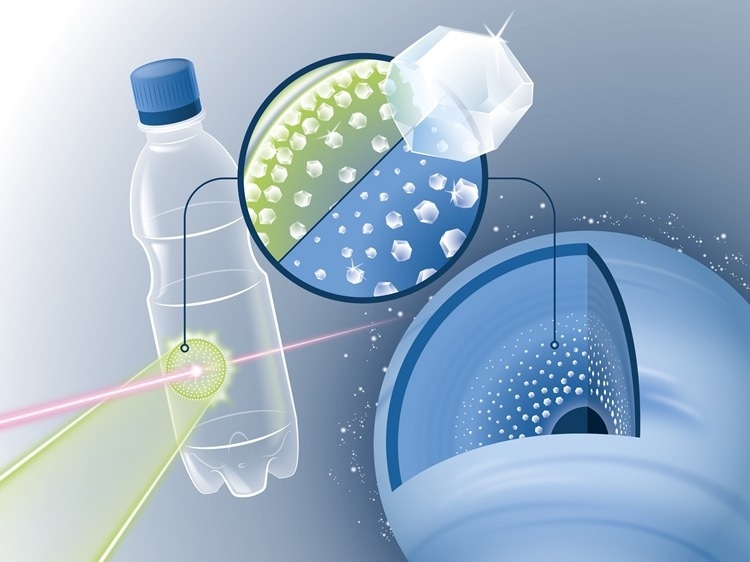 In the experiment, a thin sheet of simple PET plastic was shot at with a laser. The strong laser flashes that hit the foil-like material sample briefly heated it up to 6000 degrees Celsius and thus generated a shock wave that compressed the material to millions of times the atmospheric pressure for a few nanoseconds. The scientists were able to determine that tiny diamonds, so-called nanodiamonds, formed under the extreme pressure. Image Credit: Blaurock/HZDR.
In the experiment, a thin sheet of simple PET plastic was shot at with a laser. The strong laser flashes that hit the foil-like material sample briefly heated it up to 6000 degrees Celsius and thus generated a shock wave that compressed the material to millions of times the atmospheric pressure for a few nanoseconds. The scientists were able to determine that tiny diamonds, so-called nanodiamonds, formed under the extreme pressure. Image Credit: Blaurock/HZDR.
The researchers’ original hypothesis—that diamonds are truly showering inside the ice giants at the boundary of the solar system—was confirmed. The technique might serve as the foundation for a brand-new method of producing nanodiamonds, like the kind needed for very sensitive quantum sensors. The team publishes its findings in the journal Science Advances.
Extreme temperatures and pressures are present inside ice planets like Neptune and Uranus, which are thousands of degrees Celsius hotter than the earth’s atmosphere. However, these states are briefly simulated in the lab when powerful laser flashes strike a sample of a material that resembles foil.
This heats the sample to 6000 °C in a split second and creates a shock wave that temporarily compresses the material to a million times the atmospheric pressure.
So far we have carried out such experiments with foils made of hydrocarbons. We were able to determine that tiny diamonds, so-called nanodiamonds, form under the extreme pressure.
Dominik Kraus, Physicist, Helmholtz-Zentrum Dresden-Rossendorf
Dominik Kraus is a professor at the University of Rostock.
These foils could, however, only represent a small portion of the planet’s interior. Ice planets also have significant amounts of oxygen in addition to carbon and hydrogen. When choosing an appropriate film material, the decision was made to use PET, the plastic used to make standard plastic bottles.
“PET has a good ratio of carbon, hydrogen and oxygen to simulate what happens in ice planets,” elaborates Dominik Kraus.
At the SLAC National Accelerator Laboratory in California, the researchers conducted the tests. A potent, accelerator-based X-ray laser called the Linac Coherent Light Source (LCLS) is used. It can be used to investigate what happens to a PET film when powerful laser flashes strike it.
The specialists simultaneously used two measurement techniques, using X-ray diffraction to determine whether nanodiamonds were developing. They could also see how quickly and how big the diamonds got thanks to a technique called small-angle scattering.
Oxygen as an Important Helper
Due to its influence, the oxygen accelerated the separation of carbon and hydrogen and thus promoted the formation of nanodiamonds. As a result, the carbon atoms could come together better and form diamonds.
Dominik Kraus, Physicist, Helmholtz-Zentrum Dresden-Rossendorf
This supports the idea that inside ice giants, diamonds literally fall down. The findings should apply to a great number of other planets in the galaxy, n addition to Uranus and Neptune. It is now obvious that these ice giants, which were once thought to be rare exotic species, are most likely the prevalent planetary shape outside of the solar system.
The group also discovered a further hint: Water was also thought to have developed with diamonds, albeit in an odd variation.
Dominik Kraus suspects, “So-called superionic water should have formed. The oxygen atoms form a crystal lattice in which the hydrogen nuclei move freely.”
Superionic water can carry electrical currents due to the nuclei’s electrical charge, which helps the ice giants’ magnetic fields form. The working group’s investigations, however, have not yet allowed them to conclusively demonstrate the presence of superionic water in the mixture with diamonds.
This will be carried out in the future at the European XFEL in Hamburg, the most potent X-ray laser in the world, in close collaboration with the University of Rostock. The international user consortium HIBEF, which is led by the HZDR there, will provide the best conditions for such tests.
Nanodiamond Precision Factory
The new experiment brings up possibilities for a technical application, the targeted creation of diamonds with a diameter of less than one nm, in addition to these more fundamental discoveries. These nanodiamonds are now utilized in polishing and grinding agents. Future applications include highly sensitive quantum sensors, medicinal contrast agents, and effective reaction accelerators, such as those used to split CO2.
So far, such diamonds have mainly been produced by detonating explosives. With the help of laser flashes, they could be manufactured much more cleanly in the future.
Dominik Kraus, Physicist, Helmholtz-Zentrum Dresden-Rossendorf
Ten light flashes per second are fired by a high-performance laser onto a PET film, which is scanned by the beam every tenth of a second. The reaction produces nanodiamonds, which shoot out of the foil like bullets and land in a water-filled collecting basin. They can be filtered and practically harvested there after being slowed down.
Dominik Kraus concludes, “Nanodiamonds can be specifically tailored with it, for example in terms of their size or doping with foreign atoms. Because with the X-ray laser we have a laboratory tool with which the size growth of the diamonds can be precisely controlled.”
Journal Reference:
He, Z., et al. (2022) Diamond formation kinetics in shock-compressed C─H─O samples recorded by small-angle x-ray scattering and x-ray diffraction. Science Advances. doi.org/10.1126/sciadv.abo0617