Ossification and concomitant vascularization are crucial processes for healing large bone fractures. Despite the multifunctional role of endothelial cells, vascularizing large bone grafts in engineered bone constructs remains a challenge, thereby limiting their clinical translation.
Study: Injectable nanoporous microgels generate vascularized constructs and support bone regeneration in critical-sized defects. Image Credit: Naeblys/Shutterstock.com
An article published in Scientific Reports demonstrated an easy method to prepare vascularized constructs based on microgels that promoted human mesenchymal stromal cell (MSC) osteogenesis and supported sprouting angiogenesis and network formation.
The enzymatically degradable microgels showed a high rate of hydration and polymer density comparable to those of natural skeletal tissues. Atomic force microscopy (AFM) was used as an indentation tool to measure Young’s modulus (189 kilopascals), which was favorable for osteogenesis and vasculogenesis.
The bone matrix-mimicking osteogenic microgel was composed of gelatin, chitosan, and hydroxyapatite, which promoted the attachment and growth of MSCs. Alternatively, gelatin-containing vasculogenic microgels enabled the formation of vascular networks when integrated into 3D matrices and enriched with endothelial cells.
Combining osteogenic and vasculogenic microgels facilitates the generation of a hybrid construct for osteogenesis and vasculogenesis. Furthermore, injecting osteogenic microgels in animal models resulted in bone regeneration with >95% defect closure within 12 weeks.
Clinical Practices for Bone Healing Process
Despite the bone’s regenerative ability, large bone fractures undergo slow, improper, or incomplete healing, sometimes leading to an ability to heal, causing pseudarthrosis. Within this framework, there exists an urgent need to address these concerns caused by perturbing injuries, tumor resection-associated bone loss, and failed arthroplasties.
While a range of grafting materials are used to fix the fractures, conventional autologous grafts were adopted clinically for surgical reconstruction due to minimal risk of rejection and fast healing. However, incorporating these grafts may cause donor-site morbidities and intraoperative blood loss.
Recently, bone tissue engineering has played a crucial role in healing bone defects. Despite their success, the application of tissue engineering approaches and prostheses is limited due to complicated surgical procedures, long recovery times, and loss of vascularization or infection.
Utilizing the innate power of osteopotent cells by either providing them from endogenous or exogenous sources can make bone tissue engineering a feasible technology. While endogenous recruitment is a challenging process, exogenous delivery of cells is a more reliable technique resulting in predictable outcomes.
Since MSCs can regenerate and are available from autologous tissue sources, their application in bone repair has been studied extensively. Due to the long-term survival issues of implanted MSCs inside the host, clinical studies have revealed the efficiency of material-based systems in delivering MSCs and enhancing their bone regenerative potential.
Additionally, the physical properties of the materials play a significant role in determining how an MSC turns out. In tandem, scaffold designing and construction could be an effective strategy to maximize the therapeutic potential of MSCs in bone deformities.
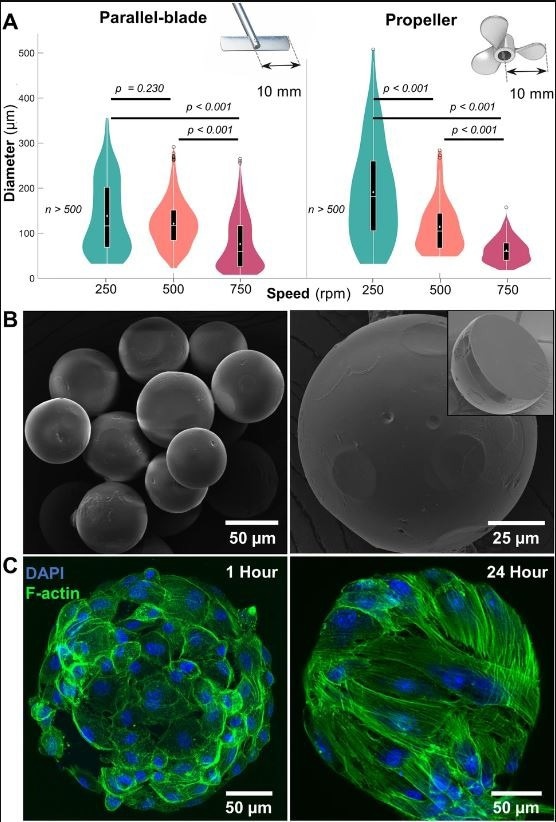
Microgel fabrication and characterization. (A) Size distribution of gelatin microgels made using straight-blade and propeller impellers at mixing speeds of 250, 500, and 750 rpm (n ≥ 500). Violin plot: White dots and lines represent mean and median, respectively. Black boxes, whiskers, and circles represent interquartile ranges, 25/75 quartile ranges, and outliers, respectively. (B) Scanning electron micrographs of dehydrated microgels with reduced size and a smooth outer surface. (Inset) Cross-section of a dehydrated microgel showing the core with a uniform, densely packed matrix. (C) Confocal micrographs of hydrated gelatin microgels seeded with mesenchymal stem cells showing a stretched cytoskeleton within 24 h. © Patrick, M.D., Keys, J.F., Suresh Kumar, H. Annamalai, R.T. (2022).
Nanoporous Microgels in Tissue Engineering
The hypo-immunogenicity, self-renewal, and differentiation abilities of MSCs make them a reliable source for tissue engineering applications. The present study demonstrated an easy method to synthesize injectable microgels to facilitate the osteogenesis of MSCs and enable the formation of an endothelial network. The microgels used in this study were fabricated from gelatin, chitosan, and hydroxyapatite.
Gelatin adds strength and cell-binding domains, chitosan resembles glycosaminoglycans, and hydroxyapatite adds rigidity to the matrix. Alternatively, vasculogenic microgels were fabricated using pure gelatin to form vascular networks and were enriched with endothelial cells.
Because gelatin undergoes sol-gel transition at 30 degree Celsius, the gelatin matrix can stabilize it under physiological conditions and facilitate the integration of other materials. The cross-linking of the matrix also influences its swelling ratio, mechanical properties, antigenicity, and cytotoxicity.
Furthermore, the composition and physical parameters of the microgels influenced osteogenesis. The high polymer density and nanoporous structure of the microgels originating from the cross-linked polypeptides enabled high water retention of the matrix, mimicking that of the bone matrix.
Conclusion
In summary, the present study demonstrated that microgels augment the functionality of MSC and endothelial cells and fabricate a construct of vascularized bone. The microgel matrix is more advantageous than other bone grafts because of its ability to mimic tissue-specific functions with minimally invasive delivery.
Experiments in animal models have shown that microgels promote the repair of critical-sized lesions and bone regeneration. To regenerate vascularized and interfacial tissues, this study has set the groundwork for establishing design principles for multiphasic scaffolds with tissue-specific biophysical and biochemical features.
Reference
Patrick, M.D., Keys, J.F., Suresh Kumar, H. Annamalai, R.T. (2022). Injectable nanoporous microgels generate vascularized constructs and support bone regeneration in critical-sized defects. Scientific Reports. https://www.nature.com/articles/s41598-022-19968-x
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.