Despite the efficiency of chemotherapy in treating primary tumors, it may induce undesirable metastasis because of proinflammatory factors being released from impaired cells, upregulating inflammatory reactions.
Study: Therapeutic Nanocarriers Inhibit Chemotherapy-Induced Breast Cancer Metastasis. Image Credit: Design_Cells/Shutterstock.com
An article published in Advanced Science presented polymeric nanoparticles that can concurrently scavenge proinflammatory factors and deliver chemotherapeutics to inhibit chemotherapy-induced metastatic breast cancer.
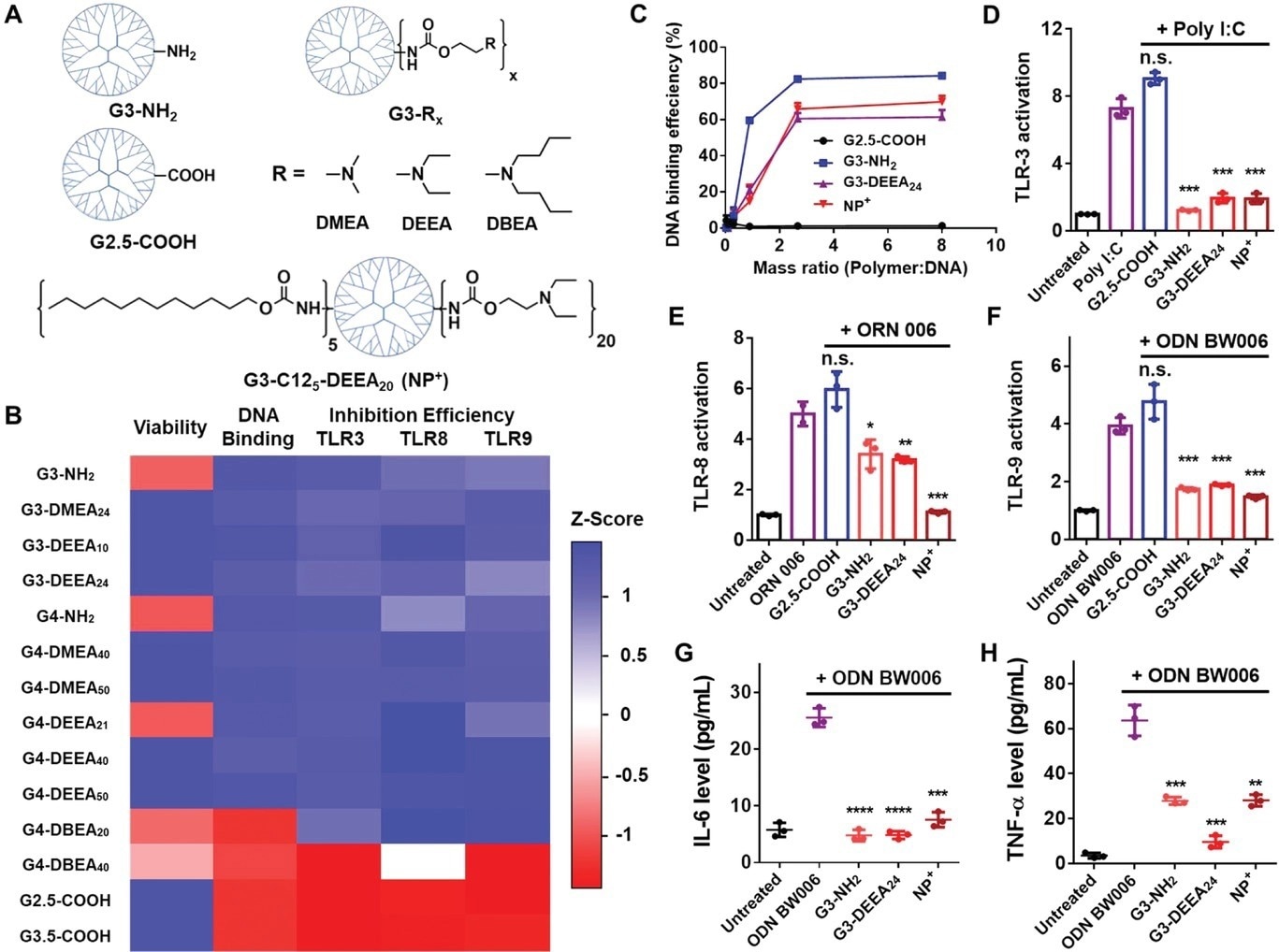
Figure 1. Structures and characterization of the PAMAM dendrimer derivatives. A) Top: Chemical structures of the PAMAM derivatives tested initially. Bottom: Structure of G3-C125-DEEA20, which was selected after additional optimization for the preparation of the nanoparticles. “NP+” is used as an abbreviation for these cationic nanoparticles. B) Heatmap of the Z scores for the different attributes of the PAMAM derivatives. Higher Z scores (blue) indicate better cell viability, DNA binding, or TLR3/8/9 inhibition. C) DNA-binding efficiency of different PAMAM derivatives and G3-C125-DEEA20 nanoparticles at different polymer:DNA mass ratios. D–F) TLR3/8/9 activation assays showing inhibition of nucleic acid agonist-induced TLR activation by the nanomaterials (polymers or nanoparticles). The DNA agonist used to activate HEK-Blue hTLR cells is listed at the top of each panel. The mass ratio of nanomaterials to agonist was 2:1. G,H) IL-6 and TNF-α levels released by RAW 264.7 cells treated with the agonist ODN BW006 with or without the addition of nanomaterials. Comparisons were made with the agonist only group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Student's t-test. Data represent the mean ± SD. © Li, T et al. (2022).
Charge–charge interactions between cationic nanoparticles and cell-free nucleic acids (cfNAs) facilitated the adsorption of the latter by the former, resulting in the downregulation of toll-like receptors (TLRs) and reduced release of inflammatory cytokines.
Modifying polyamidoamine (PAMAM) cationic dendrimers with dodecyl and diethylethanolamine surface groups (PAMAM-G3-C125-DEEA20) endowed the dendrimers with desirable properties such as nanoparticle size, reduced cytotoxicity, drug loading capacity, anti-inflammatory activity, and cfNA binding ability.
Compared to treatment with pure paclitaxel (PXT), PXT-loaded nanoparticles showed reduced levels of inflammatory cytokines and cfNAs in the blood serum of a mouse model with breast cancer metastasis, inhibiting primary tumor growth and metastasis. Moreover, the drug-loaded nanoparticles did not show any significant side effects in the serum or other organs, confirming their biosafety.
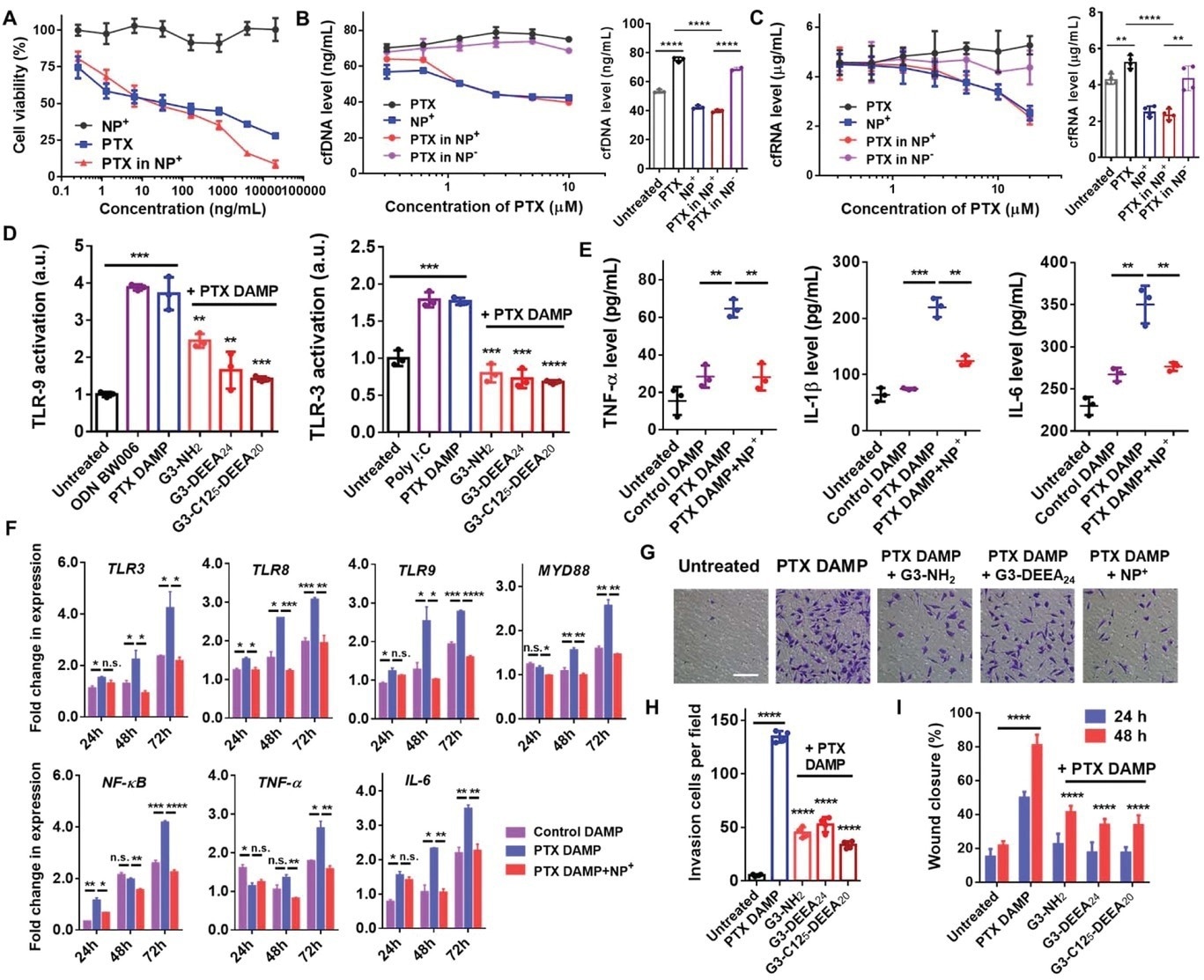
Figure 2. Nanoparticle-mediated inhibition of paclitaxel/DAMP-induced cancer cell migration and invasion. A) Viability of MDA-MB-231 cells treated with PTX only, G3-C125-DEEA20 NPs (NP+), or PTX-loaded NPs+ for 72 h. Concentrations indicate the amount of PTX in the PTX only or PTX-NP+ samples. B,C) Cell-free DNA (left) and cell-free RNA (right) levels in the supernatants of MDA-MB-231 cells treated with PTX only, NPs+, PTX-loaded NPs+, or PTX-loaded NPs− (anionic COOH-modified NPs) for 72 h. D) TLR3/9 activation assays showed that the cationic NPs inhibited PTX DAMP-induced TLR activation. PTX DAMPs—damage-associated molecular patterns caused by paclitaxel treatment—were obtained by collecting the medium of MDA-MB-231 cells treated with PTX followed by PTX removal and incubation for 72 h. Comparisons were made with the PTX DAMP only group. E) TNF-α, IL-1β, and IL-6 levels in THP-1 cells treated with DAMPs and NPs+. F) Fold changes in the expression of genes in TLR-related pathways. RNA was extracted from THP-1 cells treated with DAMPs and NPs+ for 24, 48, or 72 h. Expression level ratios were normalized to those of the untreated groups. G) Transwell experiment showing invading MDA-MB-231 cells. Scale bar, 100 µm. H) Quantification of cell invasion. Cell numbers in each 20× field were counted. Comparisons were made with the PTX DAMP only group. I) Wound healing experiment showing migrating MDA-MB-231 cells and quantitation of the wound areas. Comparisons were made with the PTX DAMP only group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Student's t-test. Data represent the mean ± SD. © Li, T et al. (2022).
Nanoparticles for Cancer Treatment
Breast cancer is amongst the most lethal cancer among females, with the second highest cancer mortality rates. Conventional treatment methods like surgery, radiotherapy, and chemotherapy are not effective enough as expected and suffer concerns about low bioavailability, low cellular uptake, emerging resistance, and adverse toxicities.
Although gene therapy using free nucleic acids has the potential to deal with key candidate genes of breast cancer, their effect is retarded due to poor cell uptake and instability in circulation. Hence, the rapidly evolving field of nanomedicine aiming for targeted drug delivery curtailing breast cancer promises to overcome the limitations of conventional therapies.
Chemotherapeutics, including doxorubicin and PTX, are frequently used in combinational therapy against primary breast cancer. Besides their efficiency in treating primary tumors, they promote metastasis by elevating proinflammatory cfNAs that are released into the tumor microenvironment (TME) by damaged cells. Addressing the chemotherapy-induced prometastatic effects in breast cancer is therefore necessary.
The cfNAs induce chronic inflammation by increasing the expression of TLRs, thereby upregulating nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) and myeloid differentiation primary response 88 (MYD88) to promote the release of inflammatory cytokines.
The unique physicochemical and technological properties of nanoparticles have allowed for several successful applications in cancer treatment. In general, nanoparticles can be optimized to improve drug bioavailability by increasing their absorption through enhanced solubility or facilitating their passage through biological membranes.
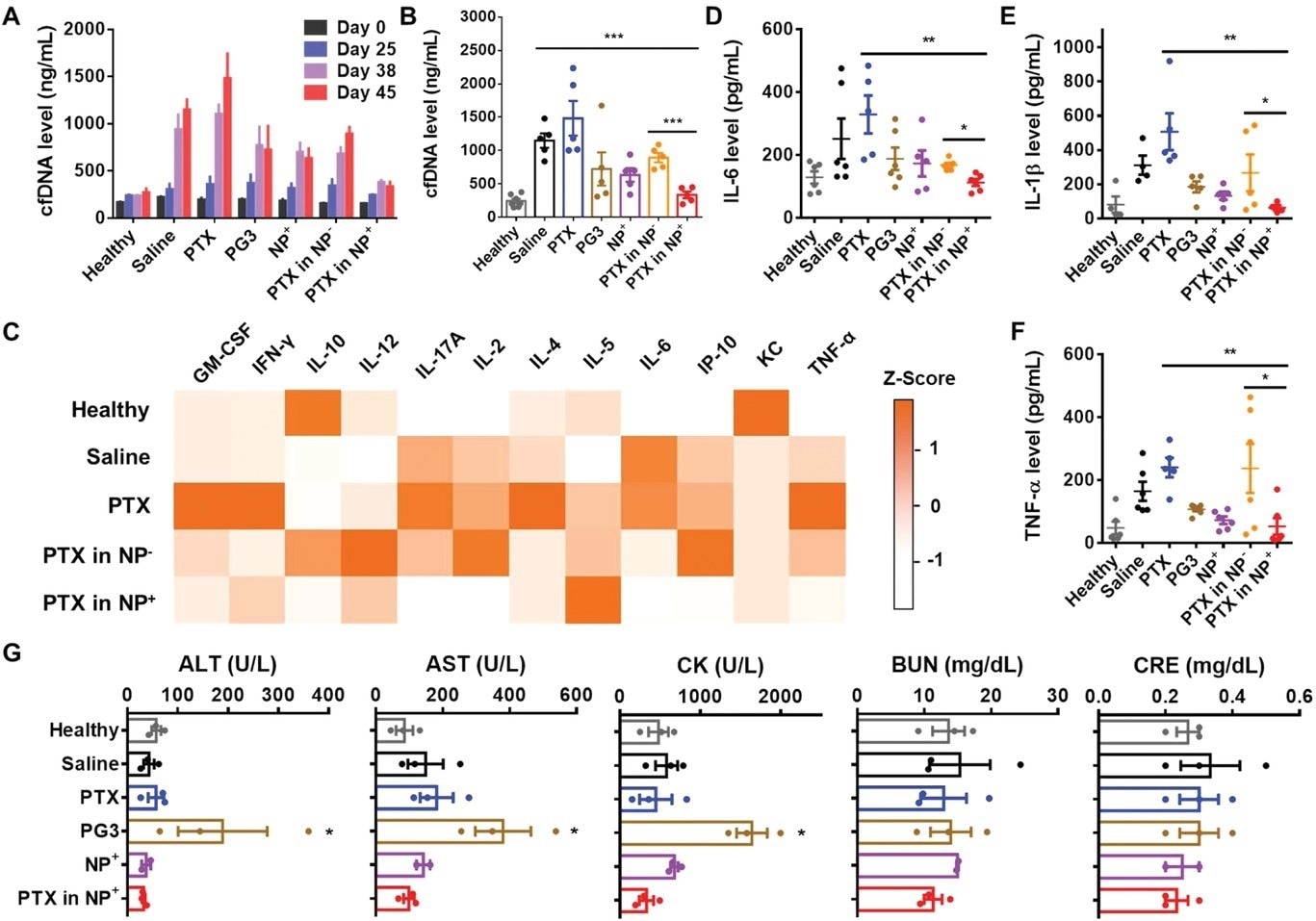
Figure 3. Inflammatory cytokine and biochemical analyses of mouse serum. A) Time-dependent cfDNA levels in the serum of BALB/c mice bearing 4T1 tumors. B) Cell-free DNA levels in mouse serum immediately after the mice were sacrificed. C) Inflammatory cytokine levels (Z scores) in mouse serum detected with IsoPlexis chips. Only detectable cytokines are shown. D–F) IL-6, IL-1β, and TNF-α levels in mouse serum detected by ELISA. G) Serum biochemical tests showing the levels of ALT, AST, CK, BUN, and CRE. Comparisons were made with the healthy group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; Student's t-test. Data represent the mean ± SD. © Li, T et al. (2022).
PTX-Loaded Cationic Nanoparticles Towards Breast Cancer Metastasis
Previous studies have described the toxic effects of PAMAM. Although PAMAM-G3 polymers have been used to scavenge cfNAs, the hydrophilic nature of PAMAM-G3 restricts its application in chemotherapeutics, necessitating further modifications.
PAMAM-based nanoparticles were fabricated in this study to administer chemotherapeutic treatment and prevent the prometastatic inflammation caused by chemotherapy. Their structures were tuned to limit cytotoxicity while realizing cfNA binding and high drug-loading capabilities.
The cationic G3-C125-DEEA20 polymer was used as a chemotherapeutic nanocarrier because of its TLR inhibition, DNA-binding ability, small size, and low toxicity. Moreover, G3-C125-DEEA20 nanoparticles could bind to cfNAs both intra- and extracellularly. Intracellular trapping of cfNAs by these cationic nanoparticles reduced the concentration of cfNAs inside the TME.
Previous studies on G3-C125-DEEA20 nanoparticles have reported their ability to prevent PTX-induced metastasis. Moreover, the in vitro studies on these nanoparticles showed a reduction in levels of cfNA in PTX damage-associated molecular pattern (DAMP) media, reducing TLR activation. PAMAM-G3-C125-DEEA20 nanoparticles were thus fabricated with the best combination of the previously mentioned properties.
The in vivo studies conducted in the present study, choosing metastasis-inducing 4T1 cells, revealed that PTX caused more metastasis than saline alone, while treatment with cationic nanoparticles induced less metastasis than PTX and saline, individually.
In contrast, PTX-loaded anionic nanoparticles inhibited the growth of primary tumors. However, they could neither scavenge cfNAs nor inhibit tumor metastasis, indicating the ability of cationic nanoparticles to scavenge cfNAs and inhibit tumor metastasis. These findings agree with previous studies using a pancreatic cancer murine model.
Conclusion
Overall, the present study developed chemotherapeutic nanocarriers to deliver the drugs and inhibit chemotherapy-induced breast cancer metastasis. Although the mice in the cationic polymer and nanoparticle therapy groups had initial tumors of sizes like those in the saline-only group, the results showed that metastatic spread was reduced in these groups.
Here, the researchers These findings demonstrate that when mice are treated with cationic polymers or nanoparticles, the metastatic spread can be inhibited, even in the presence of initial tumors. This nanocarrier strategy improves the therapeutic effects of chemotherapy by reducing the risk of metastasis, which is pertinent to numerous cancer models.
Reference
Li, T et al. (2022). Therapeutic Nanocarriers Inhibit Chemotherapy-Induced Breast Cancer Metastasis. Advanced Science. Available at: https://doi.org/10.1002/advs.202203949
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.