Reviewed by Mila PereraOct 31 2022
A unique two-dimensional (2D) nanoconfinement approach has been proposed to improve the oxygen evolution reaction (OER) activity of metal-organic frameworks (MOFs) with low conductivity. The results of the study have been published in the journal Nature Communications.
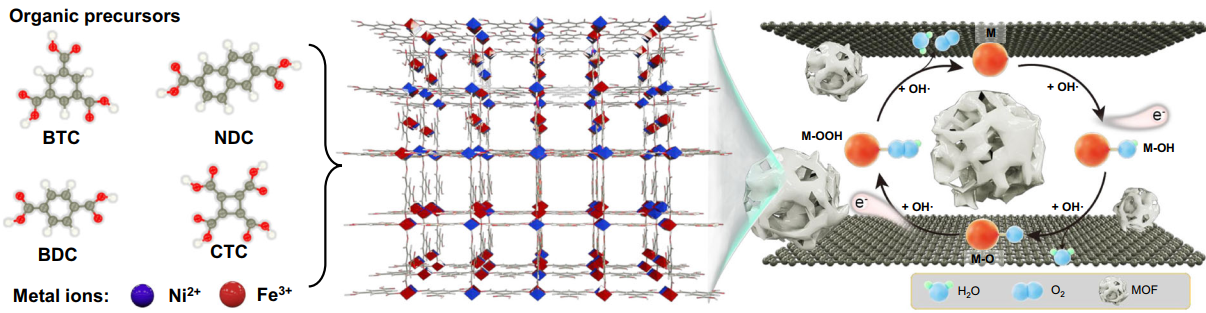
The as-prepared NiFe-MOF//G via the nanoconfinement from graphene multilayers. Image Credit: Ningbo Institute of Materials Technology and Engineering (NIMTE)
This novel approach was put together by Professor Zhang Tao's group at the Ningbo Institute of Materials Technology and Engineering (NIMTE) of the Chinese Academy of Sciences (CAS), in partnership with Professor Xiao Jianping from the Dalian Institute of Chemical Physics of CAS and Professor Hou Yang from Zhejiang University.
The progress of highly effective electrocatalysts for the electrochemical altering of water to produce eco-friendly and sustainable hydrogen energy has garnered increasing attention over the decades. Despite the crucial role the OER plays in water splitting, OER at the anode eagers for a high thermodynamic potential to accelerate water splitting kinetics.
Due to the large surface area, diverse compositions, tunable porosity, and metal centers, MOFs have risen as potential candidates for highly effective OER electrocatalysts. However, the innate poor conductivity of a majority of MOFs obstructs their catalytic performance.
To resolve this problem, scientists at NIMTE suggested an electrochemical approach to restrain MOFs between graphene multilayers through the two-electrode electrochemical system, thereby bestowing poorly conductive MOFs with robustly improved catalytic performance.
The as-prepared NiFe-MOF//G displays an unusually low overpotential of 106 mV to extend to 10 mA cm-2, exceeding the original NiFe-MOF and other previously recorded MOFs and their derivatives. Moreover, the NiFe-MOF//G electrode is highly stable and can sustain the performance for over 150 h at 10 mA cm-2 without noticeable activity decline.
The outcomes of X-Ray absorption spectroscopy tests and density-functional theory calculations show that the nanoconfinement from graphene multilayers improves the electronic structure and catalysis center of MOF materials with the development of extremely reactive NiO6-FeO5 distorted octahedral species in MOF structure.
Furthermore, the nanoconfinement minimizes the restrictive potential for the water oxidation reaction.
The nanoconfinement approach can be applied to other diverse MOFs with various structures, significantly enhancing their electrocatalytic performances. This study challenges the prevalent idea of original MOFs as inert catalysts and exposes the unlimited application prospects of poorly conductive or even insulating MOFs for electrocatalysis applications.
Journal Reference
Lyu, S., et al. (2022) Exceptional catalytic activity of oxygen evolution reaction via two-dimensional graphene multilayer confined metal-organic frameworks. Nature Communications. doi.org/10.1038/s41467-022-33847-z.