Regulated development of well-organized metal-organic framework (MOF) nanoarrays on appropriate substrates is crucial for various applications, including catalysis, sensor technologies, and optoelectronics. However, developing a simple approach for fabricating multipurpose MOF nanoarrays remains a significant research problem.
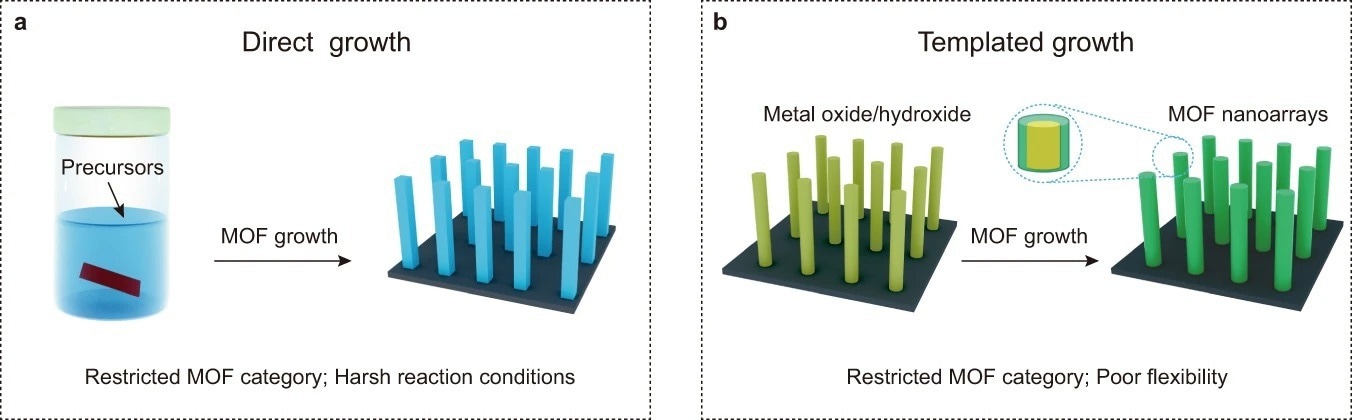
Traditional routes to MOF nanoarrays. a Direct growth of MOF nanoarrays under solvothermal conditions. b Directed growth of MOF nanoarrays on hard templates. © Wang, S., et al. (2022).
A recent study published in the journal Nature Communications focuses on this issue by presenting an extremely configurable soft nanobrush-directed technique for accurate in situ production of MOF nanoarrays on various surfaces.
Metal-Organic Framework Nanoarrays: Overview and Significance
Nanoarrays that integrate nanometer size and directional configuration have sparked considerable interest in many industries. In particular, metal-organic frameworks (MOFs) with tuneable pore size and chemical activity provide significant benefits in fabricating versatile nanoarrays.
Metal-organic frameworks (MOFs) are crystalline micro/mesoporous composite substances made of metallic ions or clusters linked together by organic linkers. Due to their distinctive structural properties, MOFs have recently emerged as novel photocatalyst materials for various catalytic applications.
The homogeneous orientation of inherently porous nanopillars produces a hierarchically inclusive environment for complete active site accessibility and free movement of reacting substrates, making MOFs suitable for catalysis, drug delivery, and energy storage applications.
MOFs, like other heterogeneous catalysts, can facilitate post-reaction separation and recyclability more than homogeneous catalysts. In certain circumstances, they also provide significantly improved catalytic stability.
Limitations of Previous MOF Nanoarrays Fabrication Methods
Precisely ordered metal-organic framework (MOF) nanoarrays can often grow readily on surfaces using "one-pot" solvothermal processes. These techniques are simple yet effective; however, they typically necessitate using MOFs with intrinsic one- or two-dimensional crystalline frameworks.
Several other techniques for growing MOFs as directed nanoarrays have also been published. However, these approaches are only applicable to the synthesis of a limited amount of MOF structures. For instance, vapor-assisted conversion (VAC) may be utilized to synthesize thin films of various MOFs.
Template-directed fabrication may also be used to create multifunctional MOF nanoarrays. Metal oxide and hydroxide arrays have been routinely exploited as hard substrates to drive the formation of MOF nanoarrays. Although these designs enhance MOF formation and reasonably guide MOF development, they often break down to liberate metal resources necessary for creating desirable MOFs.
As a result, the composition of templates significantly limits the range of MOF nanoarrays. Additionally, manipulating the height of the nanoarrays or concurrently introducing complementary chemical species in the MOFs remains a significant difficulty.
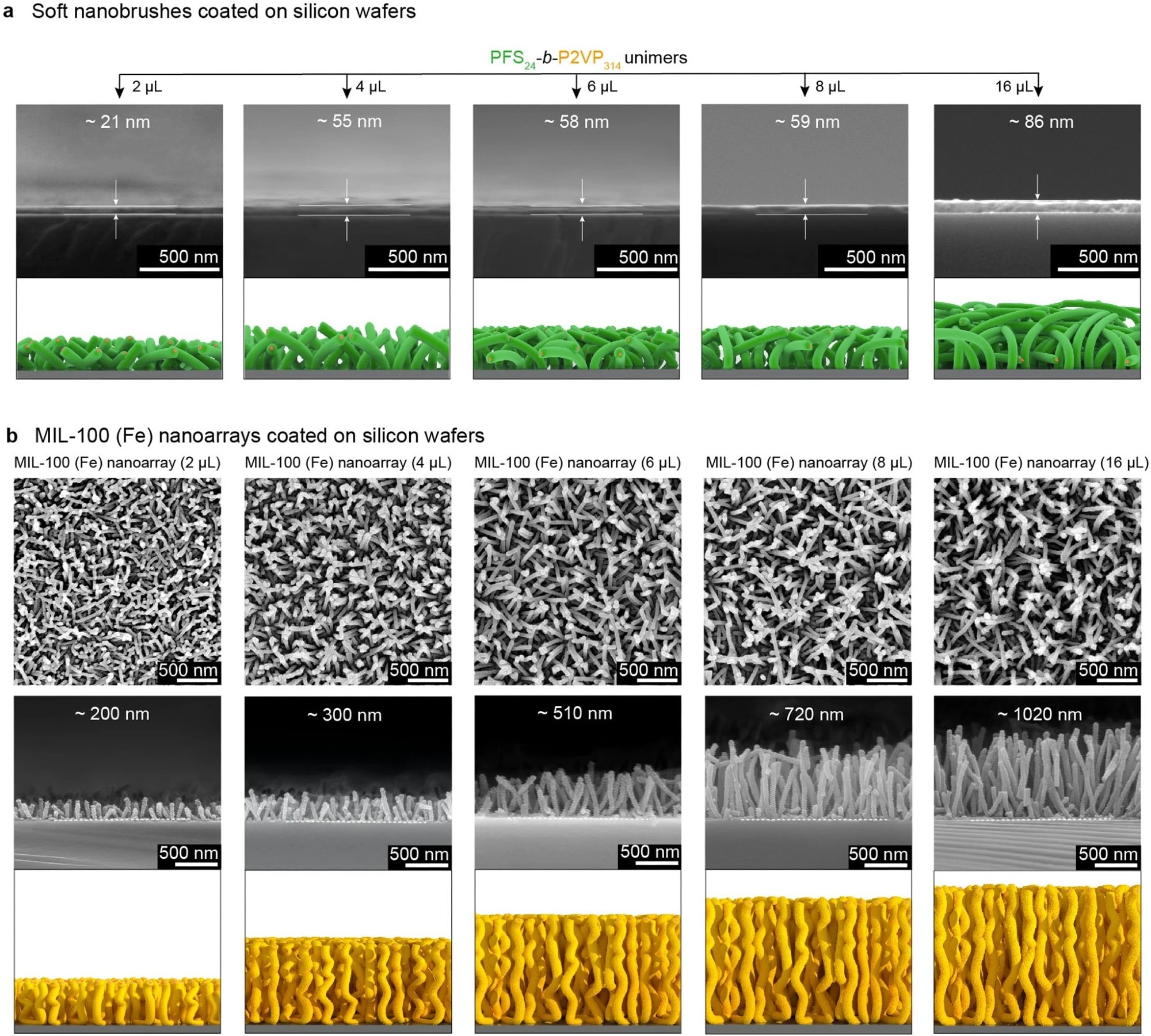
Figure 2. Height regulation of MIL-100 (Fe) nanoarrays. a Cross-sectional SEM images of soft nanobrushes formed with the addition of 2, 4, 6, 8 and 16 µL of a THF solution (10 mg/mL) of PFS24-b-P2VP314 unimers. b Top-view and cross-sectional SEM images of MIL-100 (Fe) nanoarrays directed by the corresponding soft nanobrushes shown in a. © Wang, S., et al. (2022).
Highlights of the Current Study
In this study, the researchers used an extremely versatile soft nanobrush-directed approach for the accurate production of MOF nanoarrays. The soft nanobrushes were easily placed onto different substrates by live crystallization-driven self-assembly. Their numerous pyridine sites served as catalytic sites for the absorption of many metal cations, directing the formation of distinct MOFs.
Three well-defined MOF nanoarrays with large aspect ratios and adjustable heights were formed by submerging the silicon wafer, nickel foam, and ceramic tube in specified precursor solutions. After that, noble metallic nanoparticles were meticulously inserted into the nanoarrays for simultaneous catalytic oxidation of methanol and highly-sensitive hydrogen sulfide detection.
Important Findings of the Research
The height of MOF nanoarrays could be easily controlled from 220 to 1100 nanometers by accurately manipulating the lengths of soft nanobrushes. The soft nanobrushes also offered a versatile substrate for the effective inclusion of a wide range of complementary functional species with a variety of synergistic properties.
To investigate the flexibility of the fabrication approach, the soft nanobrushes were used to guide the formation of MOF nanoarrays by continuously submerging the soft nanobrush-coated silicon wafer in various ethanol solutions. The as-prepared nanoarrays were made up of well-defined rod-like columns with a mean diameter of 45 nanometers.
MOF nanoarrays offered an exceptional platform for catalysis because of the open and free environment as well as the abundance of metallic binding sites. It should be highlighted that the as-prepared MOF nanoarrays' topology was well retained following the catalytic reaction, indicating exceptional structural stability.
MOF nanoarrays also presented a suitable platform for gas sensing due to their very high porosity and abundance of metal active sites.
Based on these results, it is anticipated that the soft nanobrush design technique presented in this study would not only ease the synthesis of various varieties of MOFs but will also provide a straightforward pathway to other inorganic multifunctional nanoarrays.
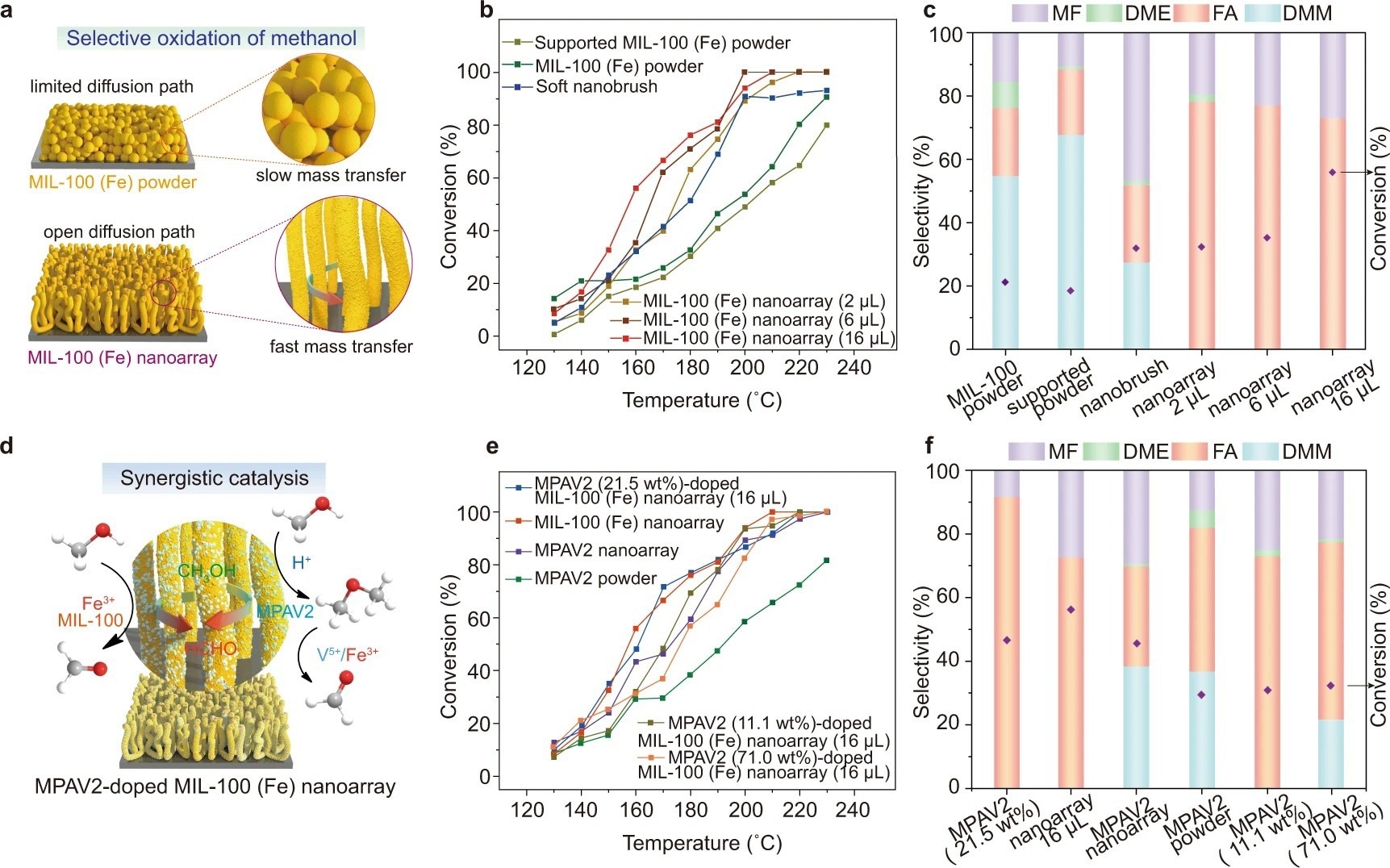
Figure 3. Selective oxidation of methanol catalyzed by MIL-100 (Fe) nanoarrays. a Schematic illustration of the catalytic environment of a MIL-100 (Fe) nanoarray and a layer of MIL-100 (Fe) powder. b, c Temperature dependence of conversion (b) and FA selectivity (c) at 160 °C for the oxidation of methanol in the presence of the pristine MIL-100 (Fe) powder, MIL-100 (Fe) powder immobilized on a silicon wafer, soft nanobrushes and MIL-100 (Fe) nanoarrays (2 µL, 6 µL, 16 µL), respectively. d Schematic illustration of the synergistic catalytic effect between the MIL-100 (Fe) nanoarray and MPAV2 during the oxidation of methanol. e, f Temperature dependence of conversion (e) and FA selectivity (f) at 160 °C for the oxidation of methanol in the presence of the MPAV2 (21.5 wt%)-doped MIL-100 (Fe) nanoarray (16 µL), MIL-100 (Fe) nanoarray (16 µL), MPAV2 nanoarray, MPAV2 powder, MPAV2 (11.1 wt%)-doped MIL-100 (Fe) nanoarray (16 µL) and MPAV2 (71.0 wt%)-doped MIL-100 (Fe) nanoarray (16 µL), respectively. Note: DMM = dimethoxymethane, DME = dimethyl ether. © Wang, S., et al. (2022).
Reference
Wang, S., et al. (2022). Soft nanobrush-directed multifunctional MOF nanoarrays. Nature Communications. Available at: https://doi.org/10.1038/s41467-022-34512-1
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.