.jpg) By Pritam RoyReviewed by Susha Cheriyedath, M.Sc.Nov 16 2022
By Pritam RoyReviewed by Susha Cheriyedath, M.Sc.Nov 16 2022In an article published in Nature Communications, researchers created a hydrogel with nanozyme-reinforcement to alter the unfavorable rheumatoid arthritis (RA) microenvironment and enhance prosthetic interface osseointegration.

Study: Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Image Credit: Emily frost/Shutterstock.com
The synthetic nanozyme hydrogel produced dissolved oxygen through a synergistic process in addition to scavenging endogenously overexpressed reactive oxygen species (ROS).
The nanozyme hydrogel could be used as an injectable delivery medium of bone marrow-derived mesenchymal stem cells (BMSCs) due to its efficacy in protecting implanted cells from hypoxia-mediated death, ROS, and osteogenic restriction. This BMSC-encapsulated hydrogel with nanozyme-reinforcement could promote osseointegration and reduce the symptoms of rheumatoid arthritis by lowering inflammatory cytokines in the affected area.
For enhancing the efficacy of osseointegration following joint replacement in rheumatoid arthritis patients, stem cell-based therapy has shown considerable potential. However, the accumulation of ROS and inadequate oxygen supply endanger the therapeutic efficiency of this strategy.
In this work, the authors revolutionized the traditional intervention techniques for enhancing prosthetic interface osseointegration in rheumatoid arthritis and offered a solution to the persistent problem of stem cell transplantation.
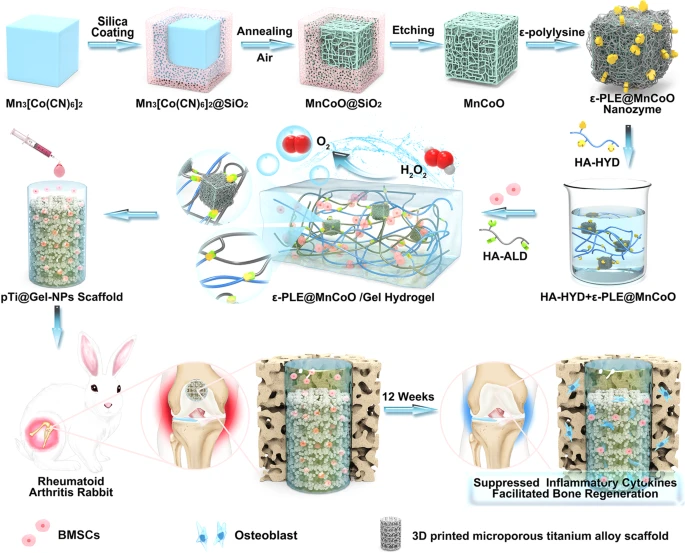
Fig. 1. Development of nanozyme-reinforced hydrogels as H2O2-driven oxygenerators to regulate stem cell behavior. Schematic diagram illustrating the synthesis process of nanozyme-reinforced self-protecting hydrogel and its application for enhancing prosthetic interface osseointegration in RA therapy.
Optimizing Stem-Cell Therapy with Nanozyme Hydrogel
Rheumatoid arthritis is among the most common and severe autoimmune diseases. It is primarily characterized by synovial membrane inflammation, which worsens over time and causes joint discomfort, rigidity, loss of functionality, and sometimes limited mobility. Thus, it necessitates joint replacement surgery to relieve pain and rehabilitate function. Despite various therapeutic techniques, the hostile rheumatoid arthritis microenvironment has not yet recovered or resolved and remains inadequate.
In contrast to conventional interventional or pharmacological therapies, stem cell-based treatments have recently gained popularity as a possible alternative for managing rheumatoid arthritis. The multipotent progenitor cells, known as BMSCs, can best differentiate into osteoblasts and chondrocytes.
BMSCs can enhance osseointegration during joint replacement surgery for rheumatoid arthritis, preventing postoperative problems, including prosthesis displacement and loosening.
Although stem cell-based therapy shows benefits for managing rheumatoid arthritis, specific insurmountable barriers prevent its widespread use. The hypoxic microenvironment of rheumatoid arthritis is one of the challenges to effective treatment.
Data shows a significant role of infiltrated inflammatory cells and fibroblast-like hyperplastic synoviocytes in the high oxygen consumption in the rheumatoid arthritis synovium. The synovium’s hypoxic situation is exacerbated by the microvasculature’s severely dysregulated design, inhibiting oxygen delivery to the area. Another unfavorable characteristic of rheumatoid arthritis is the rampant overproduction of ROS.
In this study, the authors created hydrogel reinforced with nanozyme that acted as H2O2-driven oxygeners to control stem cell behavior. The catalase-like nanozyme and a dynamically cross-linked natural polymer were the primary components of the hydrogel inspired by biological metabolism. This nanozyme hydrogel showed effective self-healing, injectable, and biocompatible features.
More notably, the developed hydrogel system could efficiently break down endogenous H2O2 to create oxygen due to the incorporation of a catalytic nanozyme. The accompanying in vitro studies showed that the nanozyme-reinforced hydrogel effectively reduced rheumatoid arthritis’s hypoxic and oxidative microenvironment, creating a suitable 3D microenvironment for BMSC osteogenesis and proliferation.
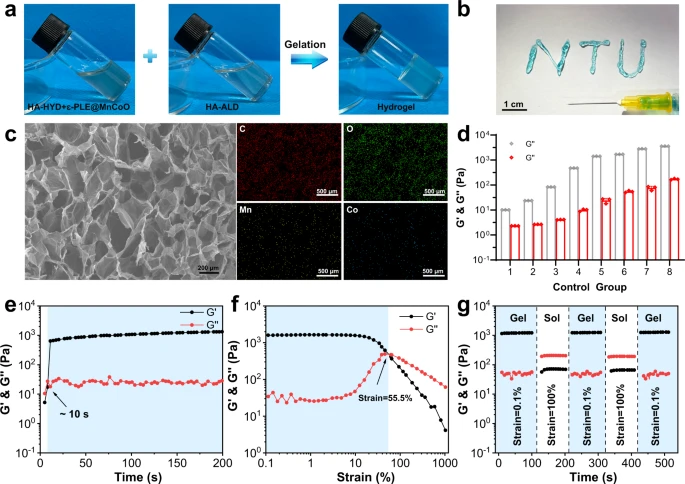
Fig. 2. Characterization of the ε-PLE@MnCoO/Gel hydrogel. a Sol-gel transition of the ε-PLE@MnCoO/Gel hydrogel. b Injectability of the ε-PLE@MnCoO/Gel hydrogel. c SEM image of the lyophilized ε-PLE@MnCoO/Gel hydrogel and corresponding elemental mapping of the synthesized hydrogel. A representative image of three replicates from each group is shown. d G’ and G” values of hydrogels containing different concentrations of polymer. Data are presented as mean values ± SD (n = 3 independent samples). e Evolution of the G’ and G” values over time for the ε-PLE@MnCoO/Gel hydrogel. f Dependence of the G’ and G” values over strain for the ε-PLE@MnCoO/Gel hydrogel. g Step strain measurements of the ε-PLE@MnCoO/Gel hydrogel with a fixed frequency of 10 rad s−1. Each strain interval was kept as 100 s. Source data are provided as a Source Data file.
Proof-of-Concept Investigations
The self-protecting nanozyme hydrogel was purposefully designed as a delivery vehicle for improving prosthetic interface osseointegration in rheumatoid arthritis to control stem cell behavior in the rheumatoid arthritis microenvironment. Also, the development of cellular behaviors was significantly influenced by the mechanical properties of the nanozyme hydrogel. Thus, comparative research on the mechanical characteristics of the hydrogel with polymer content was done.
The hydrogels’ ability to heal itself promised to provide modified stem cells with long-lasting protection, resulting in excellent therapeutic efficacy. Hydrogel treatments for bone tissue were likewise highly reliant on biodegradability. A quantitative analysis of the shift in dry weight of the hydrogel was further explored under physiological settings to investigate the hydrogel in vitro degradation capacity.
The hydrogel-mediated stem cell treatments against rheumatoid arthritis were subsequently evaluated in vivo, encouraged by favorable biocompatibility, increased proliferation, and enhanced osteogenic differentiation in vitro. Hence, the certification was performed in a severe rheumatoid arthritis rabbit model to demonstrate the benefits.
Also, the cell-laden, nanozyme hydrogel was injected into the enormous micropores of the pTi@Gel-NPs group, a 3D-printed titanium alloy prosthesis. Two other control groups were built to study the effects of various variables. These groups included the original 3D-printed microporous titanium alloy scaffold loaded with BMSCs called the pTi group, and pTi modified with BMSCs present in the gel, i.e., the pTi@Gel group.
The prosthetic interface osseointegration was enhanced and inflammatory cytokines were successfully suppressed by the nanozyme-reinforced hydrogel-containing stem cells. These results demonstrated the designed hydrogel scaffold’s considerable potential for improving the effectiveness of stem cell treatment in rheumatoid arthritis.
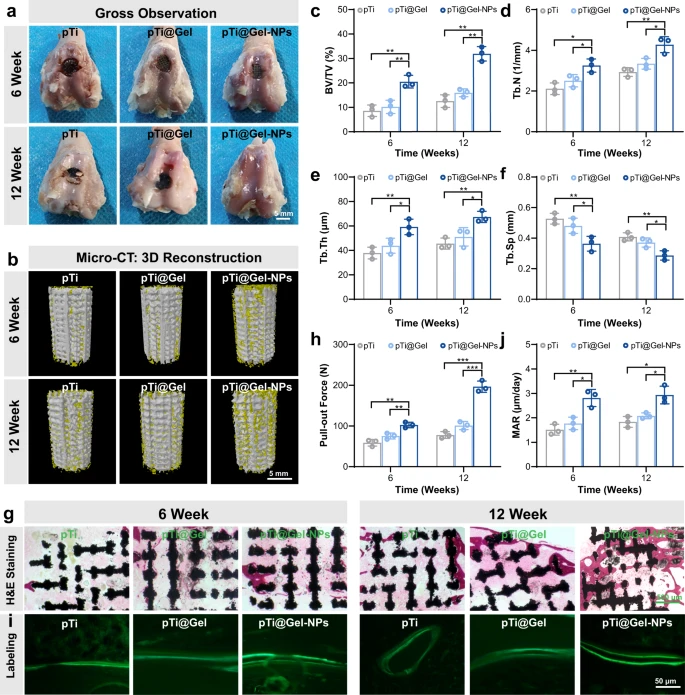
Fig. 3. Prosthetic interface osseointegration by hydrogel-mediated stem cell therapy. a Gross appearance for the articular surface of distal femurs at weeks 6 and 12 after the treatment of pTi, pTi@Gel, and pTi@Gel-NPs scaffolds. b Representative 3D reconstruction images of bone regeneration from different scaffold treatments. c–f Quantitative statistics of BV/TV (c), Tb.N (d), Tb.Th (e), and Tb.Sp (f) from different groups according to Micro-CT scanning. g H&E staining of bone defects at weeks 6 and 12 after the treatment. h Quantitative analysis of the osseointegration according to biomechanical pull-out test. i Calcein fluorescence double-labeling staining of bone defects at weeks 6 and 12 after the treatment. j Quantitative analysis of mineral apposition rate via calcein fluorescence double-labeling. These data are presented as mean values ± SD (n = 3 independent experiments). Statistical significance was determined by two-tailed t test. *P < 0.05, **P < 0.01, and ***P < 0.001. Source data and exact P values are provided as a Source Data file.
Significance of the Study
Rheumatoid arthritis is a chronic inflammatory disease that impairs joint function gradually and irreversibly. Although stem cell-based therapies have been regarded as a potential method for treating rheumatoid arthritis, their therapeutic efficiency is still severely hampered by the excessive ROS production and the inadequate oxygenation of the rheumatoid arthritis pathogenic microenvironment.
In this work, the authors introduced a hydrogel inspired by biological metabolism to reshape the unfavorable rheumatoid arthritis microenvironment. The novel hydrogel was constructed of a naturally occurring, dynamically cross-linked polymer with the metal-organic framework (MOF)-derived nanozyme. This modified nanozyme hydrogel demonstrated exceptional self-healing capacity, improved injectable performance, and biocompatibility.
Notably, the nanozyme-reinforced hydrogel shielded the implanted BMSCs from hypoxia-mediated death and ROS due to its ability to break down ROS and create oxygen synergistically.
The H2O2-driven oxygenerator could be used in addition to the nanozyme hydrogel’s self-protecting function as a cell delivery system to promote stem cell survival, osteogenic differentiation, and proliferation in vitro.
The possibility for immunomodulation was examined using a rabbit model of rheumatoid arthritis and nanozyme hydrogel-mediated stem cell treatment. In rheumatoid arthritis, the infiltration of inflammatory cells was linked to the destruction of subchondral bone tissue and erosion of the articular cartilage.
Rheumatoid arthritis patients eventually needed joint replacement surgery to reduce pain and restore joint function, particularly in the knee joint, where poor joint function resulted in limited mobility. Improvement of bone osseointegration and regeneration was highly desired for joint replacement in rheumatoid arthritis patients to prevent various postoperative problems.
A robust therapeutic impact was produced by neutralizing inflammatory substances, which locally slowed the rate of total bone deterioration and enhanced osteogenic activity. The authors postulated that pTi@Gel-NPs could improve prosthetic interface osseointegration in a rheumatoid arthritis rabbit model, considering the synergistic immunomodulation of nanozyme hydrogel-mediated stem cell treatment.
The study’s findings supported the hypothesis that the nanozyme hydrogel-mediated stem cell treatment enabled pTi@Gel-NPs to achieve improved bone regeneration efficacy and excellent osteointegration. The cell-laden hydrogel reinforced with nanozyme was a potent solution to lessen displacement, loosening, and periprosthetic fractures following prosthesis implantation in rheumatoid arthritis. A viable strategy was proposed for stem cell therapy, which had many potential applications for treating various immune-related disorders in addition to rheumatoid arthritis.
Reference
Zhao, Y et al. (2022). Nanozyme-reinforced hydrogel as a H2O2-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nature Communications. https://www.nature.com/articles/s41467-022-34481-5
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.