PNRs are ribbon-like strands of the 2D material phosphorous, which like graphene, are composed of layers of atoms that are only single-atom thick. They were produced in 2019 by a group headed by UCL Professor Chris Howard after more than a hundred theoretical studies predicted that they would have a variety of exciting and beneficial characteristics.
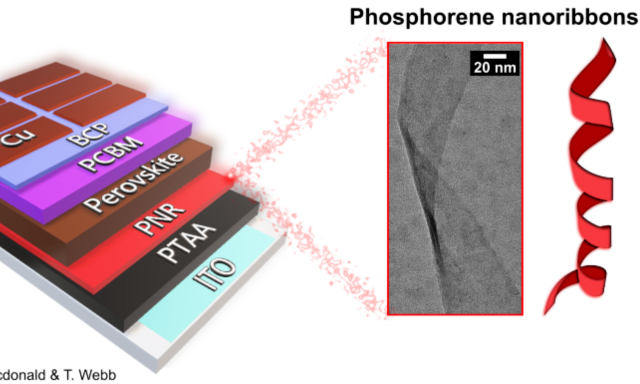 Phosphorene nanoribbons in a solar cell. Image Credit T. MacDonald/T.Webb
Phosphorene nanoribbons in a solar cell. Image Credit T. MacDonald/T.Webb
PNRs immediately found a place in their first energy device, solar cells, in 2021, directed by Dr. Tom Macdonald of Queen Mary University of London/Imperial College London and backed by the UCL team.
Dr. Tom Macdonald demonstrated how PNRs may be easily printed as an additional layer to improve solar cell functioning and efficiency by increasing “hole mobility.” Because “holes” are the opposing partner of electrons in electrical transport, increasing their mobility (the rate at which they pass through the material) allows electrical current to move more effectively between device layers.
QMUL and UCL have collaborated once more to establish a vision for how PNRs might be utilized to help address the energy problem.
This is exciting as we discuss how PNRs could have an essential role to plan in our race against climate change. Last year we showed that PNRs can be printed onto perovskite solar cells to improve their efficiency; and allow low-cost printing into thin, flexible films compared to traditional inflexible silicon-based solar cells. Dr. Macdonald, Research Fellow, Queen Mary University of London
Macdonald continues, "The promise of our PNR solar cells is incredible, but just the start of the many areas PNRs can revolutionize, from lithium-ion batteries to generating clean hydrogen gas."
In their Joule perspective, Dr. Macdonald emphasizes the important steps that scientists all over the world have already taken to develop and use PNRs. This includes studies revealing that integrating PNRs into lithium-ion batteries significantly improves stability and performance, with the PNRs able to suppress the formation of unwanted dendrites that develop from the surface of the negative electrode.
Dendrites may puncture the separator and make contact with the battery’s cathode material. This causes electronic contact between the positive and negative electrodes, resulting in lithium-ion battery instability.
Check out our interview with Dr. Macdonald here.
Journal Reference:
Macdonald, T. J., et al. (2022) Phosphorene nanoribbons for next-generation energy devices. Joule. doi.org/10.1016l/j.joule.2022.09.010.