Researchers at Rice University and the University of Texas at Austin observed that when it comes to packing charge-acceptor molecules on the surface of semiconducting nanocrystals, more is not better.
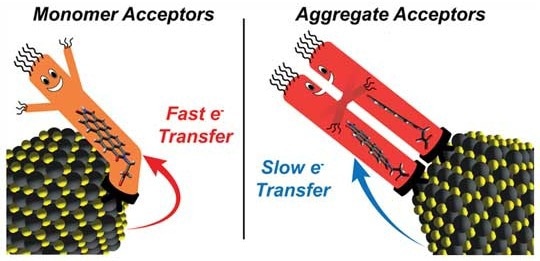 Chemists from Rice University and the University of Texas at Austin showed that adding more charge-accepting ligands to the surface of semiconducting nanocrystals can produce ligand-ligand interactions that reduce the rate of electron transfer in hybrid nanomaterials. Image Credit: P. Rossky/Rice University
Chemists from Rice University and the University of Texas at Austin showed that adding more charge-accepting ligands to the surface of semiconducting nanocrystals can produce ligand-ligand interactions that reduce the rate of electron transfer in hybrid nanomaterials. Image Credit: P. Rossky/Rice University
Hybrid nanomaterials can be designed to capture, detect, convert, or control light in specific ways by combining organic and inorganic elements. The rate of scientific publication about these materials has increased more than tenfold over the past 20 years due to the high level of interest in them.
For instance, they could increase the effectiveness of solar power systems by capturing energy from sunlight at wavelengths, such as infrared, which is not captured by conventional photovoltaic solar panels.
The materials are made by combining “charge acceptor” molecules, which serve as ligands and attach to the surface of semiconductors to transport electrons away from the semiconductor nanocrystals, with nanocrystals of light-capturing semiconductors.
The most-studied nanocrystal systems feature high concentrations of charge acceptors that are bound directly to the semiconducting crystals. Generally, people try to maximize the surface concentration of charge acceptors because they expect the rate of electron transfer to continuously increase with surface-acceptor concentration.
Peter Rossky, Study Co-Corresponding Author and Professor, Department of Chemistry, Rice University
According to a few studies, electron transfer rates initially rise with the concentration of surface acceptors before falling as surface concentrations continue to rise. Rossky and co-corresponding author Sean Roberts, an associate professor of chemistry at UT Austin, were aware that interactions between the molecular orbitals of ligands could affect charge transfer.
They predicted that there would come a time when adding more ligands to a crystal’s surface would result in these interactions.
The National Science Foundation (NSF)-funded Center for Adapting Flaws into Features (CAFF), based at Rice University, seeks to use microscopic chemical flaws in materials to create cutting-edge catalysts, coatings, and electronics. Rossky and Roberts are two of the program’s co-principal investigators.
Rossky, Roberts, and colleagues at CAFF conducted a thorough investigation of hybrid materials containing lead sulfide nanocrystals and varying concentrations of the frequently researched organic dye perylene diimide (PDI) to test their hypothesis.
The experiments demonstrated that a gradual increase in PDI concentration on nanocrystal surfaces eventually led to a sharp decline in electron transfer rates.
The influence of ligand-ligand interactions between PDI molecules on the geometries of PDI aggregates on crystal surfaces, according to Rossky, is the key to the behavior.
Each research team had to contribute their expertise and use a careful combination of spectroscopic experiments, electronic structure calculations, and molecular dynamics simulations to put together the evidence needed to demonstrate the significance of these aggregation effects.
Our results demonstrate the importance of considering ligand-ligand interactions when designing light-activated hybrid nanocrystal materials for charge separation. We showed ligand aggregation can definitely slow electron transfer in some circumstances. But intriguingly, our computational models predict ligand aggregation can also speed electron transfer in other circumstances.
Sean Roberts, Associate Professor, Chemistry, University of Texas at Austin
In addition to holding the Harry C. and Olga K. Wiess Chair in Natural Sciences at Rice, Rossky is a professor of chemistry and chemical and biomolecular engineering.
The NSF (CHE-2124983, CNS-1338099, DGE-1610403) and the Welch Foundation (F-1885, F-1188) provided funding for the study. The National Institutes of Health (OD021508) has supported the instrumentation while Advanced Micro Devices Inc. and Rice’s Center for Research Computing have supported high-performance computing.
Journal Reference
Cadena, D. M., et al. (2022) Aggregation of Charge Acceptors on Nanocrystal Surfaces Alters Rates of Photoinduced Electron Transfer. Journal of the American Chemical Society. doi:10.1021/jacs.2c09758.