Reviewed by Alex SmithJan 12 2023
Pyroelectric catalysis (pyro-catalysis) can transform temperature fluctuations in the environment into clean chemical energy such as hydrogen. However, pyro-catalysis is ineffective when compared to more typical catalysis strategies, like photocatalysis, due to slow temperature fluctuations in the ambient environment.
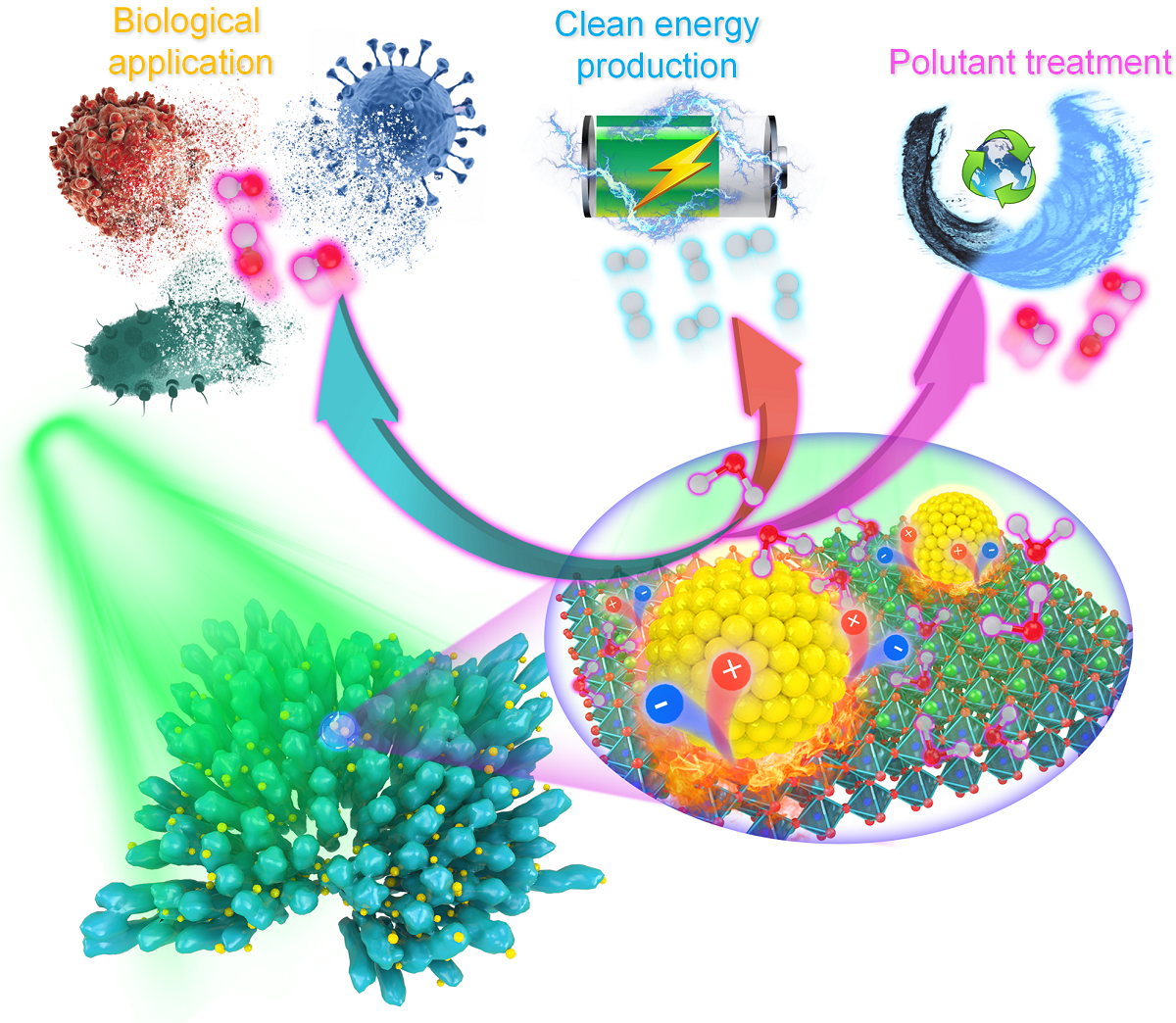 Illustration of potential applications of combining pyroelectric materials and the localized thermo-plasmonic effect of noble metal nanomaterials. Image Credit: Dr Lei Dangyuan’s group/City University of Hong Kong
Illustration of potential applications of combining pyroelectric materials and the localized thermo-plasmonic effect of noble metal nanomaterials. Image Credit: Dr Lei Dangyuan’s group/City University of Hong Kong
Recently, a group led by City University of Hong Kong (CityU) investigators activated a substantially faster and more efficient pyro-catalytic reaction by employing targeted plasmonic heat sources to quickly and efficiently heat up and cool down the pyro-catalytic material. The results pave the way for more effective catalysis in biological applications, pollutant treatment, and the production of clean energy.
The catalysis induced by surface charges in pyroelectric materials caused by temperature changes is referred to as pyro-catalysis. It is an environmentally friendly, self-powered catalytic process that recovers waste thermal energy from the environment. It is gaining popularity in the production of clean energy and the formation of reactive oxygen species, which may then be utilized for disinfection and dye treatment.
However, most currently available pyroelectric materials are inefficient if the ambient temperature does not change significantly over time. because the rate of environmental temperature change is generally limited, increasing the number of temperature cycling is a more practical strategy to boost pyro-catalytic efficacy.
However, achieving numerous thermal cycling in the pyro-catalyst within a short time interval using traditional heating methods is a significant difficulty.
Challenge of Multiple Thermal Cycling
Dr Lei Dangyuan, Associate Professor in the Department of Materials Science and Engineering (MSE) at CityU, recently directed a research group that tackled this problem by merging pyroelectric materials and the localized thermo-plasmonic effect of noble metal nanomaterials.
Plasmonic nanostructures, which enable the collective oscillation of free electrons, can absorb light and rapidly convert it to heat. Its nanoscale size enables rapid but effective temperature adjustments inside a limited container while minimizing heat loss to the surrounding environment. As a result, the thermo-plasmonic nanostructures’ localized heat may be readily fine-tuned and turned on or off by external light irradiation in an ultrashort time interval.
In their research, the researchers used barium titanate (BaTiO3) nanoparticles, a prominent pyro-catalytic material. As plasmonic heat sources, the coral-like BaTiO3 nanoparticles are coated with gold nanoparticles; the gold nanoparticles may directly convert photons from a pulsed laser to heat.
The experiment's findings showed that gold nanoparticles operate as a fast, dynamic, and controllable localized heat source without boosting the surrounding temperature, significantly and efficiently increasing the overall pyro-catalytic reaction rate of BaTiO3 nanoparticles.
Gold Nanoparticles as a Localized Heat Source
The group produced a high pyro-catalytic hydrogen production rate using this approach, expediting the practical application development of pyro-catalysis. Through thermo-plasmonic local heating and cooling under the irradiation of a nanosecond laser at 532 nm, the plasmonic pyroelectric nano-reactors displayed an increased pyro-catalytic hydrogen production rate of around 133.1 ± 4.4 µmol·g-1·h-1.
Additionally, the nanosecond laser employed in the experiment had a repetition rate of 10 Hz, which indicated that ten pulses of light were irradiated on the catalyst each second to achieve ten heating and cooling cycles. This means that the pyroelectric catalytic performance could be increased in the future by raising the laser pulse repetition rate.
By developing a novel pyroelectric composite system with other photothermal materials, the study team feels that their experiment results have opened up a new strategy for increasing pyro-catalysis. This significant advancement makes using pyro-catalysis in pollutant treatment and clean energy production possible in the future.
The findings were published in the journal Nature Communications.
Dr. You Huilin, from The Hong Kong Polytechnic University (PolyU), and Dr. Li Siqi, then-postdoc in Dr. Lei’s group are the co-first authors of the study. The corresponding authors are Dr. Lei and Professor Huang Haitao, from PolyU. Other team members include Dr. Fan Yulong, from the MSE at CityU, and collaborators from PolyU.
The study was funded by the Research Grants Council of Hong Kong and the National Natural Science Foundation of China through the Excellent Young Scientists fund.
Journal Reference:
You, H., et al. (2022) Accelerated pyro-catalytic hydrogen production enabled by plasmonic local heating of Au on pyroelectric BaTiO3 nanoparticles. Nature Communications. doi.org/10.1038/s41467-022-33818-4.