Image Credit: Ritsumeikan University
In a recent study, researchers from Japan were able to create LH nanorings by allowing chlorophyll derivatives to self-assemble. They then investigated the environmental factors that contributed to their development. Their results could open the door to new materials for LH devices like solar cells and aid research into artificial photosynthesis.
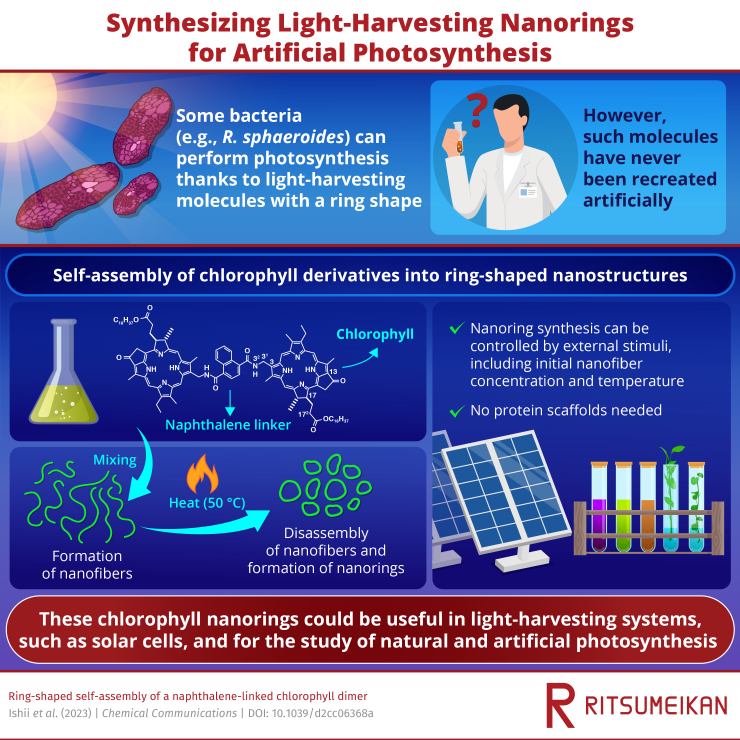
The sun is the source of almost all chemical energy used by life on Earth. This is because plants and some types of bacteria (usually at the base of the food chain) may employ light-harvesting (LH) supramolecules (two or more molecules linked together by intermolecular interactions) to propel photosynthesis.
These supramolecules require many pigments, including chlorophyll, organized in unique configurations that differ between species to function.
For instance, LH antennae are seen in green photosynthetic bacteria, where spiral structures made of chlorophyll molecules eventually assemble into large tubular supramolecules. In contrast, purple photosynthetic bacteria like Rhodobacter sphaeroides have several LH antennae types with ring-shaped structures made of chlorophyll pigments.
While the tubular chlorophyll aggregates have been successfully artificially created in the lab using a self-assembly method, their ring-shaped counterparts have not.
A group of scientists from Japan was able to fill this information gap in recent research that was published in Chemical Communication on January 26th, 2023. They found that combining naphthalenediamide with a derivative of chlorophyll in an organic solvent produced dimers that spontaneously self-assembled into rings with a diameter of several hundred nanometers.
Professor Hitoshi Tamiaki of Ritsumeikan University and Assistant Professor Shogo Matsubara of Nagoya Institute of Technology were members of the team.
The team was taken aback by their initial discovery and set out to learn more about how the ring-shaped nanostructures were made and how they functioned.
They discovered that chlorophyll dimers, molecules made of two chlorophyll units connected by naphthalene, first self-assembled into stable wavy nanofibers after further examination using atomic force microscopy.
These nanofibers broke down into smaller nanoring precursors at a temperature of 50 °C, and their ends were finally connected to create the required nanorings.
It is interesting to note that this nanofiber-to-nanoring transition required external stimuli. Dimer concentration and temperature were shown to be important factors.
At low concentrations, ring-shaped aggregates were obtained by a preferential end-to-end joining of a single fiber supramolecule. In contrast, end-to-end linkage between different nanofibers was prevalent at higher concentrations and gave rise to network nanostructures.
Hitoshi Tamiaki, Professor, College of Life Sciences, Department of Applied Chemistry, Ritsumeikan University
Overall, this study’s findings point to a simple method for producing the LH supramolecule, which has long confounded researchers.
The self-assemblies we synthesized enable efficient sunlight absorption along with excitation energy migration and transfer. Mimicking the arrangement of chlorophyll pigments observed in nature is critical to not only understand natural photosynthesis but also construct artificial LH systems for devices such as solar cells.
Shogo Matsubara, Assistant Professor, Graduate School of Engineering Tsukuri College, Nagoya Institute of Technology
Furthermore, the structural transformation from nanoring to nanofiber brought on by inputs from external stimuli could assist in the development of new smart materials with tunable features.
The group mentioned that more research is being done on the optical characteristics of the self-assembled nanorings.
Journal Reference:
Ishii, T., et al. (2023) Ring-shaped self-assembly of a naphthalene-linked chlorophyll dimer. Chemical Communication. doi:10.1039/D2CC06368A.