A fuel cell is a type of electric power generator that can convert hydrogen gas into energy while only emitting water as waste. It is envisaged that this extremely effective clean energy system will be essential in the adoption of the hydrogen economy, taking the place of power plants and combustion engines and batteries in vehicles and trucks.
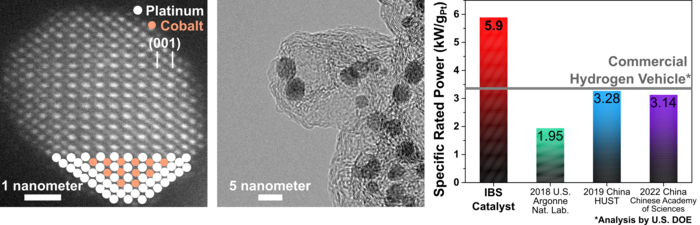
Microscopic (STEM and TEM) images of the developed catalyst and its PEMFC power performance. Compared to the state-of-the-art fuel cell catalysts, the catalyst developed by the CNR-IBS team showed almost twice the power performance per platinum use. Image Credit: Institute for Basic Science
However, the price of platinum, which could reach as high as 30,000 USD per kg, has been a significant obstacle, making fuel cell catalysts unaffordable. High-performance catalyst manufacturing techniques have likewise proved difficult and substantially constrained.
As a result, it is necessary to create a simple and scalable process for producing platinum-based fuel cell catalysts, and also to improve catalytic performance and stability while utilizing the least amount of platinum possible.
To address this problem, a research team from the Center for Nanoparticle Research (CNR) at the Institute for Basic Science (IBS) in South Korea, led by professors Yung-Eun Sung and Taeghwan Hyeon, has found a brand-new technique for manufacturing nanocatalysts.
These evenly sized (3–4 nm) cobalt–platinum (Co–Pt) alloy nanoparticles were generated by simple thermal treatment, according to the researchers. The impregnation method’s simplicity of synthesis and the colloidal method’s fine control over the size and form of the nanocrystals are combined in this procedure.
Two oppositely charged metal complexes, Co and Pt ions encircled by bipyridine and chlorine ligands, make up the unique Co-Pt alloy nanocatalysts created by the CNR-IBS team.
The study team proposed that the bipyridine ligand would thermally decompose creating a carbon shell that can shield the developing Co-Pt alloy nanoparticles using a simple heat procedure.
They were successful in producing a highly homogeneous nanocatalyst with nanoparticles that were just 3–4 nm in size after improving the heat treatment conditions.
The intermetallic phase, in which the unstable Co atoms are stabilized by the surrounding Pt atoms, is how Co and Pt atoms were organized in the nanocatalyst that the researchers created.
Also, ionomers (proton conductors) were uniformly distributed throughout the whole catalyst layer in the fuel cell when nitrogen was successfully doped onto the carbon support. This improved the delivery of oxygen gas to the surface of the Co-Pt nanocatalyst.
The proton-exchange membrane fuel cell demonstrated a significantly improved power performance with a high specific rated power of 5.9 kW/gPt, which is almost double that of the current performance in a commercial hydrogen vehicle.
Most of the 2025 objectives established by the US Department of Energy (DOE), with the objective of steady long-term fuel cell operation have been met by the catalyst created by the team.
The CNR-IBS team is convinced that this research will encourage the production of fuel cell catalysts of maximum sophistication. These discoveries would also aid in enhancing the endurance and catalytic efficiency of alloy nanocatalysts for a variety of additional electrocatalytic uses.
Design of a novel bimetallic compound as a precursor material has been the critical starting point in this study. We have developed a platform technology to produce a complicated form of alloy nanocatalysts through a simple and scalable method, and finally achieved an enhanced fuel cell power performance with less amount of platinum used.
Taeghwan Hyeon, Professor, Center for Nanoparticle Research, Institute for Basic Science
Prof. Yung-Eun Sung further added, “A world-class level of fuel cell performance has been achieved in this research, surpassing most of the 2025 targets of US DOE by lessening the amount of platinum that can contribute up to around 40% of the cost of fuel cells. We expect that this study, together with some follow-up studies, would greatly impact the growth of the hydrogen vehicle industry and the realization of hydrogen economy in the near future.”
Journal Reference
Yoo, T. Y., et al. (2023) Scalable production of an intermetallic Pt–Co electrocatalyst for high-power proton-exchange-membrane fuel cells. Energy and Environmental Science. doi:10.1039/D2EE04211H.