Small interfering RNAs (siRNAs) are new therapeutics that can be used to treat a variety of diseases. This has increased the need for safe, effective, and targeted methods of siRNA delivery in cells. Researchers from the University of Amsterdam and Leiden have now created customized molecular nanocages for siRNA delivery.
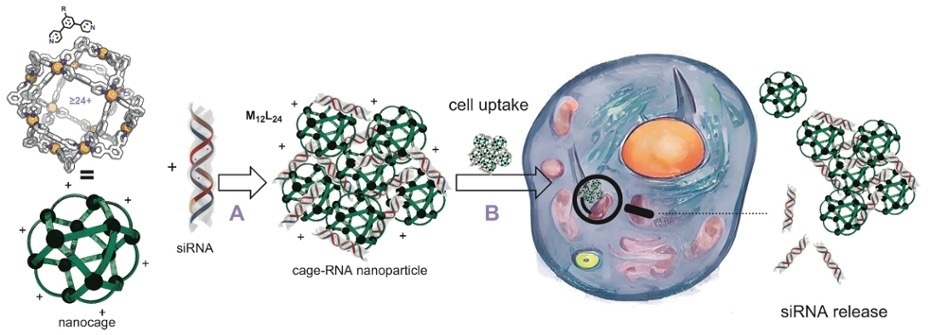
Schematic representation of the use of nanocages for siRNA delivery. When binding siRNA (arrow A), the nanocages with a typical diameter of 5-6 nanometer cluster into cage-RNA nanoparticles with a diameter of some 150 nanometers. This is small enough to enter a cell (arrow B) and deliver the siRNA. Image taken from the paper. Image Credit: University of Amsterdam
They presented easy-to-prepare nanocages with adjustable siRNA delivery features in a study published in the journal Chem.
The homogeneous, supramolecular, and bio-inspired catalysis research team led by Prof. Joost Reek and Prof. Bas de Bruin at the University of Amsterdam’s Van’t Hoff Institute for Molecular Sciences developed the nanocages.
Prof. Alexander Kros’ group at the Leiden Institute of Chemistry continued the studies. The researchers were motivated by the potential of siRNA in gene therapy, which requires effective delivery systems.
To enable the cages to bind siRNA strands, they set out to create nanocages containing functional groups on the outside. The siRNA could theoretically be released in a cellular environment as the binding is based on reversible bonds.
The scientists conducted a laboratory experiment employing multiple human cancer cells to investigate the delivery properties of their nanocages.
A Range of Nanocages
The nanocages are structures made of small molecular building blocks known as ditopic ligands that are joined together by metal atoms. The acronym M12L24 refers to a conventional cage, which has 12 metal atoms and 24 ligands. To create molecular cages with various siRNA binding affinities, the researchers developed and synthesized five distinct ligands.
They then prepared a range of siRNA binding nanocages using platinum or palladium as connecting metal. The palladium nanocages are less stable in a cellular environment, and decomposition is one of the siRNA-releasing mechanisms.
After evaluating the stability and siRNA binding abilities of the nanocage, the delivery properties were tested in experiments based on siRNA-mediated Green Fluorescent Protein (GFP) silencing.
Fluorescence measurements were taken after siRNA was successfully delivered to human GFP-expressing cells using the cages. HeLa and U2Os cell lines of the human species were both used.
Cage Composition Determines siRNA Delivery
To their astonishment, the researchers were able to show effective siRNA delivery and also found that there was a striking difference based on the metal utilized in the nanocage. A platinum-based Pt12L24 nanocage delivered siRNA to U2OS cells extremely well but had minimal effect on HeLa cells.
The Pd12L24 nanocage made of palladium, created from the same ligand building block, delivered siRNA to HeLa but not to U2OS. This distinction could not be seen in experiments using a commercially available delivery technology (lipofectamine).
The M12L24 nanocages thus introduce the possibility of tuning siRNA delivery characteristics by tuning the nanocage composition.
The researchers believe that the nanoparticles' unique cell selectivity feature is a promising contribution to targeted RNA gene material delivery, whose full potential has not yet been realized.
Even though the current results were obtained in highly controlled laboratory research, they expect that the tuneable RNA delivery of their nanocages will spawn future developments of highly desirable selective RNA nanomedicines.
Journal Reference:
Bobylev, E. O., et al. (2023) The application of M12L24 nanocages as cell-specific siRNA delivery agents in vitro. Chem. doi:10.1016/j.chempr.2023.03.018.