Efficient biological signal transmission can be achieved with the use of implantable bioelectrodes. Metal-based bioelectrodes are associated with tissue inflammation, inefficient signal transduction, and uncontrolled stability in living biological systems.
Researchers from Gwangju Institute of Science and Technology (GIST), Korea have developed graphene-based conductive hydrogels as bioelectrode materials for overcoming the challenges associated with traditional, metal-based bioelectrodes. These conductive hydrogels are injectable, skin-compatible, easy to use, and demonstrate excellent signal transmission. Moreover, their controllable degradability can allow the development of high performing, convenient bioelectrodes with advanced applicability. Image Credit: Prof. Jae Young Lee from GIST, Korea
Researchers from Korea have developed a new type of hydrogel to solve these challenges. These graphene-based conductive hydrogels (ICHs) were developed as advanced implantable bioelectrodes.
These ICHs can be used as advanced implantable bioelectrodes since they are injectable, permit in vivo degradability control, and exhibit good signal transmission.
Electronic devices known as implantable bioelectrodes transmit signals to and from living biological systems to monitor or stimulate biological activity. Such devices can be built utilizing numerous materials and processes.
The right material for performance and biocompatibility is important due to its intimate contact with living tissues. Conductible hydrogels have attracted much attention due to their flexibility, compatibility, and superior interaction ability.
The convenience of use and performance in biological systems are hampered by the lack of injectability and degradability in conventional conductive hydrogels.
Against this background, Korean researchers have now developed graphene-based conductive hydrogels with injectable and tunable degradation, advancing the design and development of advanced bioelectrodes.
The study was led by Professor Jae Young Lee of the Gwangju Institute of Science and Technology (GIST) and was published on February 24th, 2023, in the journal Small.
Traditional implantable electrodes frequently cause several problems, such as large incision for implantation and uncontrolled stability in the body. In contrast, conductive hydrogel materials allow minimally invasive delivery and control over the bioelectrode’s functional in vivo lifespan and are thus highly desired.
Jae Young Lee, Study Lead and Professor, Gwangju Institute of Science and Technology
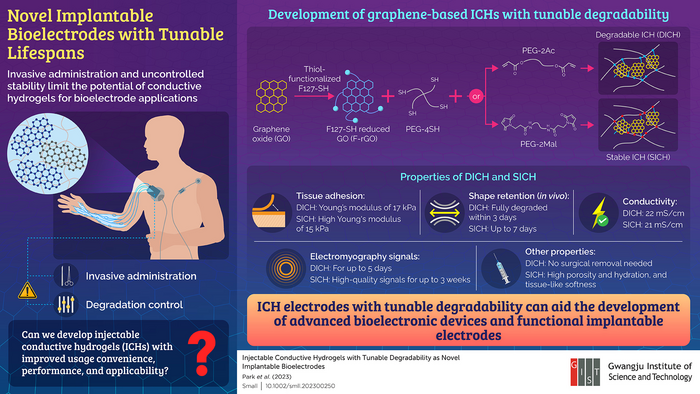
To produce the injectable conductive hydrogels (ICHs), the scientists utilized thiol-modified reduced graphene oxide (F-rGO) as the conductive element due to its expansive surface area and remarkable electrical and mechanical characteristics.
To facilitate the creation of stable and hydrolyzable ICHs, they opted for prepolymerizing dimaleimide (PEG-2Mal)- and diacrylate (PEG-2Ac)-functionalized polyethylene glycols. These prepolymerized substances were then subjected to thiol-ene reactions with poly (ethylene glycol)-tetrathiol (PEG-4SH) and F-rGO.
DICHs produced using PEG-2Ac were decomposable, whereas SICHs created with PEG-2Mal were stable. The scientists observed that the innovative ICHs excelled over numerous current ones by efficiently attaching to tissues and registering the greatest readings.
Under laboratory conditions (in a controlled environment), SICH remained stable for one month, whereas DICH exhibited progressive deterioration from the third day.
Upon being introduced to mouse skin, DICH vanished within three days of the administration, while SICH persevered in its original form for as long as seven days. Both ICHs were found to be compatible with the skin, and they displayed regulated degradability.
The team assessed the efficacy of the ICHs in capturing in vivo electromyography signals in rat muscle and skin. SICH and DICH exhibited exceptional signal quality and outperformed the conventional metal electrodes.
The SICH recordings could be monitored for three weeks, in contrast to DICH signals which became entirely undetectable within five days. These results imply that SICH electrodes are suitable for extended signal monitoring, while DICH electrodes are suitable for temporary usage that does not necessitate surgical removal.
Prof. Lee concluded, “The novel graphene-based ICH electrodes developed by us incorporate features like high signal sensitivity, simplicity of use, minimal invasiveness, and tunable degradability. Altogether, these properties can assist in the development of advanced bioelectronics and functional implantable bioelectrodes for a variety of medical conditions, such as neuromuscular diseases and neurological disorders.”
Journal Reference
Park, J., et al. (2023) Injectable Conductive Hydrogels with Tunable Degradability as Novel Implantable Bioelectrodes. Small. doi:10.1002/smll.202300250.