In an innovative exploration into the microscopic world of living cells, scientists from the National Institute for Materials Science in Tsukuba, Japan, have pioneered a technique that may revolutionize our understanding of how cells respond to external mechanical pressures. This breakthrough, led by Jun Nakanishi of the Mechanobiology Group, has been recently published in the journal Science and Technology of Advanced Materials.
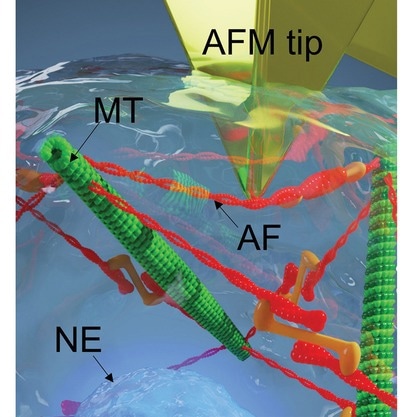
Image Credit: Wang, H. et al. (2023). Science and Technology of Advanced Materials
The 'Nano-Poke': A New Approach to Understanding Cellular Response
Utilizing atomic force microscopy (AFM), a sophisticated method that employs nanoscale probes, the research team has successfully 'poked' living cells with these microscopic tips, measuring how forces are distributed on and within the cell when force is applied. The approach employs a Markov-chain Monte Carlo optimization to refine the analysis of force-displacement curves, enhancing the sensitivity of AFM indentation.
We invented a unique way to ‘touch’ a cell with nanoscale ‘hand’, so that the force distribution over a complete cell could be mapped with nanometer resolution.
Hongxin Wang, First Author, JSPS Postdoc, Mechanobiology Group
Cells have an innate ability to respond to various environmental pressures, a process crucial for maintaining their integrity. As such, the primary aim of the investigation was to understand how cells react and adapt to mechanical stimuli.
By manipulating mechanical states, such as inhibiting myosin motor activity, the researchers observed shifts in the distribution of intracellular forces. Notably, when actin fibers' tension-bearing capacity is reduced, the cell's nucleus compensates by counterbalancing external forces. This finding signifies the dynamic adaptability of cellular structures in response to environmental changes.
Cells are smart materials that can adapt to various chemical and mechanical stimuli from their surroundings.
Jun Nakanishi, Corresponding Authors, Mechanobiology Group at the National Institute for Materials Science Lead
The research provides new insights into the cellular response mechanisms, particularly those involving external stimuli, which are essential for cell health.
These mechanisms are implicated in various diseases, such as diabetes, Parkinson's disease, heart attacks, and cancer. Until now, limitations in existing methods have hindered a comprehensive understanding of these responses.
Cancer vs. Healthy Cells: A Comparative Analysis
A fascinating aspect of the research involves comparing the responses of healthy and cancerous cells to mechanical pressure.
Intriguingly, the team observed that cancer cells exhibit greater resilience to external compression compared to healthy cells and are less prone to trigger cell death in response. This finding opens new avenues in distinguishing healthy cells from cancerous ones, potentially offering a novel diagnostic tool based on cellular mechanics.
Beyond Traditional Cancer Diagnostics
Currently, cancer diagnosis relies heavily on analyzing cell size, shape, and structure. However, these parameters are not always sufficient for accurate diagnostics. Han Zhang, another corresponding author, notes that the ability to measure force distribution offers a promising enhancement to diagnostic accuracy.
As the field of cellular mechanics continues to evolve, this research opens new avenues for understanding how cells respond to their environment.
The intricate interactions of forces within a cell, from the cytoskeleton to the nucleus, is not just a matter of structural necessity but also a critical aspect of cellular health and disease. This study, therefore, represents a significant step forward in our comprehension of these complex biological processes and marks a significant stride in the realm of nanotechnology and its application in medical science.
Journal Reference
Wang, H. et al. (2023) ‘Mapping stress inside living cells by atomic force microscopy in response to environmental stimuli’, Science and Technology of Advanced Materials, 24(1). doi:10.1080/14686996.2023.2265434.