A critical reaction in environmental chemistry is CO oxidation. Researchers have now demonstrated that the catalytic activity of a metal palladium (Pd) surface for oxidizing carbon monoxide (CO) is enhanced by porous silica sheets less than one nanometer thick.
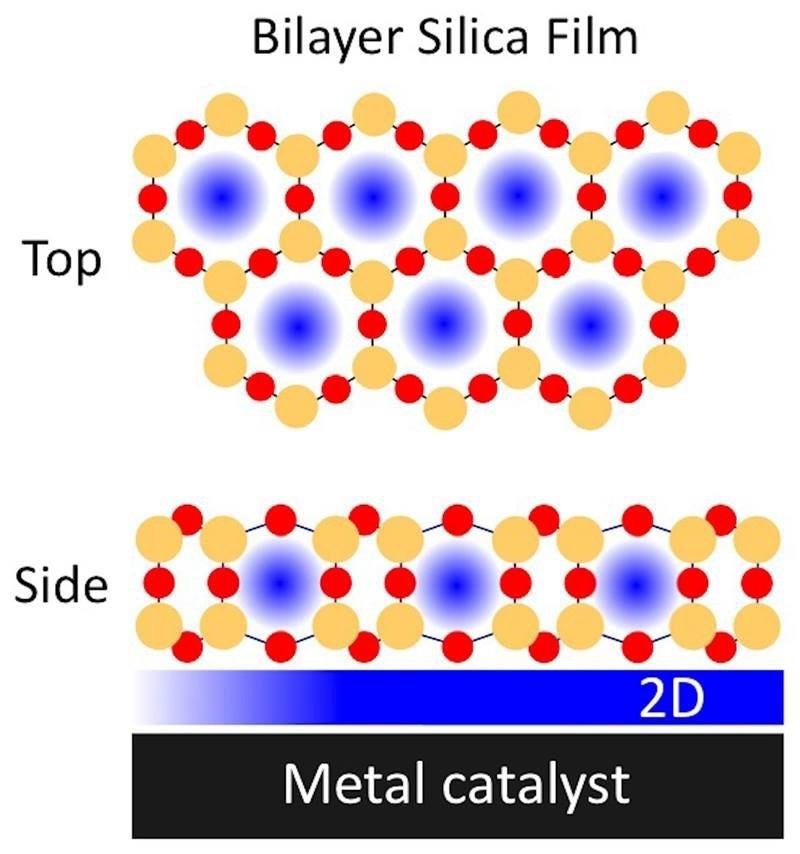 Placing a porous bilayer silica silicon dioxide (silicon in orange and oxygen in red) film on a metal catalyst enhances chemical reactions by creating confined spaces (blue) in the silica and at the silica-metal interface. Image Credit: Brookhaven National Laboratory
Placing a porous bilayer silica silicon dioxide (silicon in orange and oxygen in red) film on a metal catalyst enhances chemical reactions by creating confined spaces (blue) in the silica and at the silica-metal interface. Image Credit: Brookhaven National Laboratory
By creating materials with low concentrations of catalysts in small spaces, researchers are increasing the efficiency of catalysts. The gaps in these nanoscale materials are smaller than those of a single virus.
In this study, the researchers found that when a narrow two-dimensional gap separated the silica layer and metal catalyst, it improved CO conversion. In addition, compared to palladium alone, it resulted in a 12% increase in carbon dioxide (CO2) generation.
The Impact
CO has the ability to obstruct desirable chemical transformations and catalyze processes. The harmful CO is converted to (CO2) through carbon monoxide oxidation, usually with the help of a metal catalyst like Pd.
After that, the transformed (CO2) molecule can be readily removed from the catalyst surface and retrieved or put to various uses. These findings provide insights into the mechanisms by which 2D nanoporous silica sheets can improve CO oxidation. Other kinds of reactions can also be accelerated with this method.
Summary
The use of confined microenvironments to regulate reaction chemistry has drawn attention in the field of catalysis. A metal catalyst and a nanoporous silica bilayer material can generate a 2D nanoscale space, which can encourage different kinetic and energy schemes based on molecular-level confinement effects from this smaller volume.
To watch the CO oxidation reaction, the scientists layered the bilayer silica on top of catalytically active Pd. The researchers used mass spectrometry, in situ X-Ray and infrared spectroscopy, and in situ X-Ray spectroscopy to investigate the structure-activity connection. The structure of the silica layer was investigated, and the binding site geometry of the CO on the metal surface was determined using in situ infrared spectroscopy.
X-Ray spectroscopy monitored the surface’s gas evolution during the reaction and determined the surface's oxidation state. The study demonstrates that confinement effects imposed on surface adsorbates under 2D silica facilitate CO oxidation on Pd. Compared to Pd alone, this interaction creates a reactive surface oxide that increases CO2 production rates by 12%.
This work was made possible by the Center for Functional Nanomaterials’ (CFN) Proximal Probes Facility and the National Synchrotron Light Source-II’s (NSLS-II) In situ and Operando Soft X-Ray Spectroscopy beamline (a collaboration between the CFN and NSLS-II). The team studied the spectroscopic reaction of CO2 at functionalized and bare metal catalysts, which provided critical insight into the physics in the confined region.
The Department of Energy (DOE) Office of Science user facilities, the National Synchrotron Light Source-II, and the Center for Functional Nanomaterials provided resources for this work. The DOE Office of Science’s Basic Energy Sciences program funded the Energy Frontier Research Center, which also contributed.
Journal Reference:
Eads, C. N., et al. (2023) Enhanced Catalysis under 2D Silica: A CO Oxidation Study. Angewandte Chemie International Edition. doi:10.1002/ange.202013801