Researchers from the Research Institute of Chemical Technology, a joint center of the Spanish National Research Council (CSIC) and the Universitat Politècnica de València (UPV), as well as the ITACA Institute of the UPV, have developed a new, more cost-effective, and sustainable process for producing metal nanocatalysts.
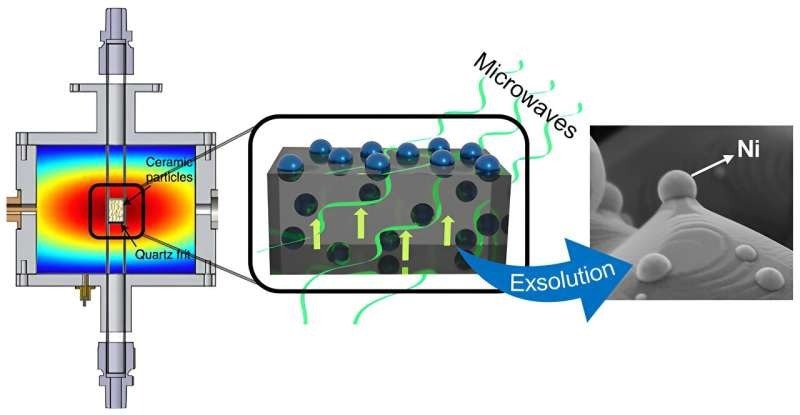
Image Credit: ACS Nano (2023). DOI: 10.1021/acsnano.3c08534
The technique has a lot of promise in the industrial sector and might help decarbonize the industry. The research has been released in ACS Nano.
The exsolution process, a method for producing metallic nanoparticles on the surface of ceramic materials, was triggered by microwave radiation and forms the foundation of this novel technique.
At elevated temperatures and in a reducing atmosphere (usually hydrogen), metal atoms migrate from the structure of the material to its surface, forming metal nanoparticles anchored to the surface. This anchoring significantly increases the strength and stability of these nanoparticles, which positively impacts the efficiency of these catalysts.
Beatriz García Baños, R&D Project Manager, ITACA Centro Tecnológico, Universitat Politècnica de València
Researchers from UPV and CSIC have demonstrated in their study that this procedure can be performed at milder temperatures and without the need to lower atmospheres, all due to microwave radiation.
In this way, active nickel nanocatalysts can be produced in a more energy-efficient exsolution process. These catalysts have been proven to be active and stable for the reaction of CO production from CO2, obtaining a product of industrial interest and contributing to the decarbonization of the sector.
Alfonso Juan Carrillo Del Teso, Researcher, Energy Conversion and Storage Group, Instituto de Tecnología Química
The traditional exsolution technique in these materials takes place at temperatures of 900 ºC and exposure lengths of around 10 hours, whereas the exsolution process exhibited in nickel nanoparticles has been carried out at temperatures of about 400 ºC and exposure times of a few seconds. Furthermore, this method makes it possible to accomplish exsolution without the need for hydrogen.
Baños added, “For all these reasons, we improve the sustainability of the process. Moreover, by obtaining the catalysts at milder temperatures and shorter exposure times, we reduce the costs of the process, which is also influenced by not having to use hydrogen as a reducing gas.”
Applications
The UPV and CSIC team’s technique is mainly meant for use in high-temperature catalytic processes that store and transform renewable energy. It might also be used in CO2 hydrogenation processes relevant to Power-to-X systems, biogas reforming reactions to generate synthesis gas (a precursor to liquid fuels), and functionalizing electrodes for fuel cells and/or high-temperature electrolyzers.
Journal Reference:
López-García, A., et. al. (2023) Microwave-Driven Exsolution of Ni Nanoparticles in A-Site Deficient Perovskites. ACS Nano. doi:10.1021/acsnano.3c08534