Using sophisticated measurement methods based on near-field optical microscopy, an interdisciplinary research team at the Institute for Molecular Science led by Jun Nishida (Assistant Prof.) and Takashi Kumagai (Associate Prof.) has successfully observed vibrational spectra of single proteins, which consist of approximately 500 amino acid residues.
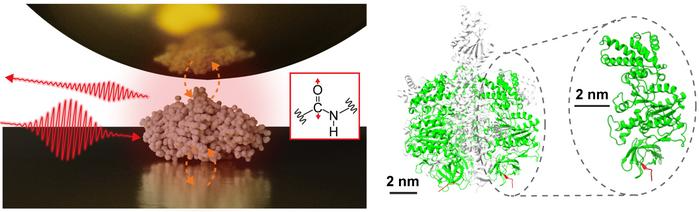 Single protein infrared vibrational spectroscopy (Left) Scheme of near-field infrared spectroscopy measuring a single protein. (Right) The structure of the protein complex F1-ATPase and the subunit measured in this study. Image Credit: Jun Nishida
Single protein infrared vibrational spectroscopy (Left) Scheme of near-field infrared spectroscopy measuring a single protein. (Right) The structure of the protein complex F1-ATPase and the subunit measured in this study. Image Credit: Jun Nishida
The accomplishment marks a significant step forward in the development of technical advancements such as ultra-sensitive and super-resolution infrared imaging, as well as single-molecule vibrational spectroscopy.
Since infrared spectroscopy can quantify vibrational spectra, often known as “molecular fingerprints,” it is frequently utilized for the structural and chemical investigation of a wide range of materials. Ultra-high sensitivity and super-resolution infrared imaging are in greater demand due to the recent fast growth of nanotechnology.
However, detecting tiny samples or obtaining spatial resolution at the nanoscale is beyond the capabilities of traditional infrared spectroscopy. It is hard to quantify a single protein, for instance, because extremely sensitive infrared microspectroscopy needs over a million proteins to produce an infrared spectrum.
The new technique makes use of light that is limited to the nanoscale, which makes it possible to analyze very small samples in detail—something that is difficult to do with traditional infrared spectroscopy.
The researchers used a gold substrate to isolate a single protein—a subunit of a protein complex known as F1-ATPase—and then conducted near-field infrared spectroscopy studies in a natural setting. They made a significant advancement by effectively obtaining the infrared vibrational spectrum of a single protein, which might help characterize the local structural organizations of specific proteins.
With improved insights into the processes and interactions of membrane proteins and protein complexes, this information is especially crucial for understanding their complicated roles. Moreover, a novel theoretical framework explaining the nanoscale interactions between the protein and the infrared near field has been created.
The group was able to statistically replicate the experimental vibrational spectra they saw by using the theory. These findings will open the door to a variety of uses for nanoscale infrared spectroscopy, including the chemical study of biomolecules and other nanomaterials.
Journal Reference:
Nishida, J., et. al. (2023) Sub-Tip-Radius Near-Field Interactions in Nano-FTIR Vibrational Spectroscopy on Single Proteins. Nano Letters. doi:10.1021/acs.nanolett.3c03479.