It might be difficult to determine the most effective way to provide chemotherapy medications to tumor cells. The treatments should ideally target tumor cells and spare healthy cells.
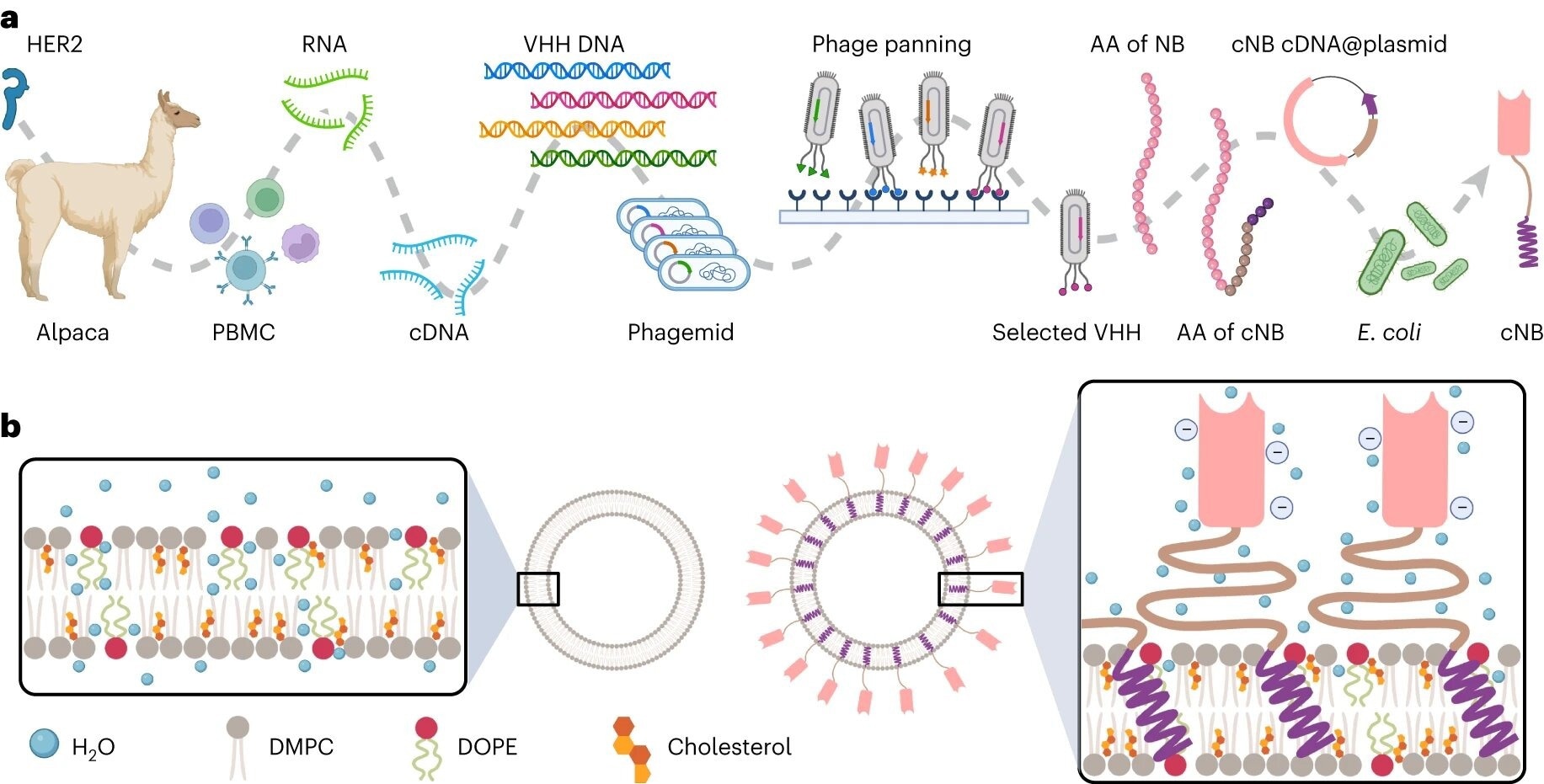 Schematic of immunoliposome preparation. a, Flowchart illustrating the manufacturing processes of cNB. Alpacas were immunized with the HER2 extracellular domain, and subsequent isolation of peripheral blood mononuclear cells (PBMCs) was performed. Total RNA was extracted from the PBMCs followed by cDNA synthesis for amplifying variable heavy domain of heavy chain (VHH) gene regions. The PCR products were then ligated into the phagemid vector, and E. coli cells were transformed with the ligated products and cultured. Colonies were recovered for the biopanning of phage displayed VHH libraries. One specific VHH was selected and sequenced to determine its amino acid sequences. Subsequently, in the design process amino acids encoding a hydrophilic linker (shown in yellow) and a STMD (shown in purple) were added to the C-terminus of the NB. The corresponding cDNA was synthesized and integrated into plasmids for the expression of the cNB using E. coli cells. b, Overview of biophysical transformation due to size, surface charge, lipid fluidity, membrane stiffness and thermostability between liposome and cNB-LP. AA, amino acid. Image Credit Nature Nanotechnology (2024). DOI: 10.1038/s41565-024-01620-6
Schematic of immunoliposome preparation. a, Flowchart illustrating the manufacturing processes of cNB. Alpacas were immunized with the HER2 extracellular domain, and subsequent isolation of peripheral blood mononuclear cells (PBMCs) was performed. Total RNA was extracted from the PBMCs followed by cDNA synthesis for amplifying variable heavy domain of heavy chain (VHH) gene regions. The PCR products were then ligated into the phagemid vector, and E. coli cells were transformed with the ligated products and cultured. Colonies were recovered for the biopanning of phage displayed VHH libraries. One specific VHH was selected and sequenced to determine its amino acid sequences. Subsequently, in the design process amino acids encoding a hydrophilic linker (shown in yellow) and a STMD (shown in purple) were added to the C-terminus of the NB. The corresponding cDNA was synthesized and integrated into plasmids for the expression of the cNB using E. coli cells. b, Overview of biophysical transformation due to size, surface charge, lipid fluidity, membrane stiffness and thermostability between liposome and cNB-LP. AA, amino acid. Image Credit Nature Nanotechnology (2024). DOI: 10.1038/s41565-024-01620-6
Immunoliposomes might be the solution. They can efficiently connect to antigens on tumor cell surfaces via their surface-targeting ligands, giving tumor cells enough time to absorb the “poison.”
The advantages of immunoliposomes in cancer therapy have been well-recognized throughout the last four decades. However, immunoliposomal drugs have not yet reached the market, despite being proven in laboratories since 1981.
Why? One significant impediment is the lack of a large-scale, low-cost, and viable production technology. Grafting targeting ligands onto plain liposomes to create immunoliposomes takes roughly a half a dozen stages and can cause complications.
A one-step production approach for immunoliposome synthesis was recently reported in the journal Nature Nanotechnology by Yuan Wan, an associate professor at Binghamton University's Thomas J. Watson College of Engineering and Applied Science. It is environmentally friendly because it does not require any chemical conjugation or necessary chemical reagents.
The traditional manufacturing process of immunoliposomes is relatively complex. It involves a lot of chemical conjugation and purification. Chemical conjugation and required reagents impair the stability and antigen binding of targeting ligands. The multistep process leads to payload leakage and product loss.
Yuan Wan, Associate Professor, Department of Biomedical Engineering, Binghamton University
Wan added, “So, immunoliposomes are less attractive to industrial manufacturers because of their low yield, high production costs, and the high risk of batch-to-batch variation. These shortcomings hinder commercial production and clinical use of immunoliposomes.”
What distinguishes Wan's study is the use of manufactured chimeric nanobodies with a “sticky” end. More than 2,500 nanobodies can be integrated into the exterior of a single 100-nanometer liposome, which is approximately 1,000 times smaller than a human hair.
This process is simpler, quicker, and cheaper than previous procedures, and it gives more influence over the final product. The surface nanobodies also create a protective barrier around the liposome, which could stop it from being eliminated by the body too rapidly and allow it to remain in circulation for longer.
Another significant advantage is that this procedure does not use harmful chemicals. Traditional techniques frequently involve a substance known as polyethylene glycol (PEG), which can occasionally create difficulties for patients, including death. Because of these concerns, the US Food and Drug Administration mandates additional monitoring for drugs containing PEG.
“Something really interesting we found is that when these chimeric nanobodies are inserted into the lipid bilayer, they actually increase the rigidity and thermal stability of the entire immunoliposomes. So, the drugs packed inside can hold up for a good 10 months without obvious leaks,” Wan stated.
Additionally, Wan is optimistic that, with additional research and clinical testing, immunoliposomes could eventually be produced and receive federal approval for clinical use, given that there are now about 20 plain liposomal drugs in use.
Wan concluded, “We are also working on developing new chimeric nanobodies to ramp up production by at least 30 times. It will make the manufacturing cost of these chimeric nanobodies much lower.”
Journal Reference:
Rahman, M. M., et. al. (2024) Chimeric nanobody-decorated liposomes by self-assembly. Nature Nanotechnology. doi:10.1038/s41565-024-01620-6.