An afterglow luminescent nanoprobe expands the potential for imaging live cells. According to a study published in Angewandte Chemie, a novel “nanotorch” could light up for more than 10 days following a single excitation. This allows microrobots’ movements through the body to be tracked in real time. Furthermore, it can be “recharged” noninvasively with near-infrared (NIR) light without the need for touch.
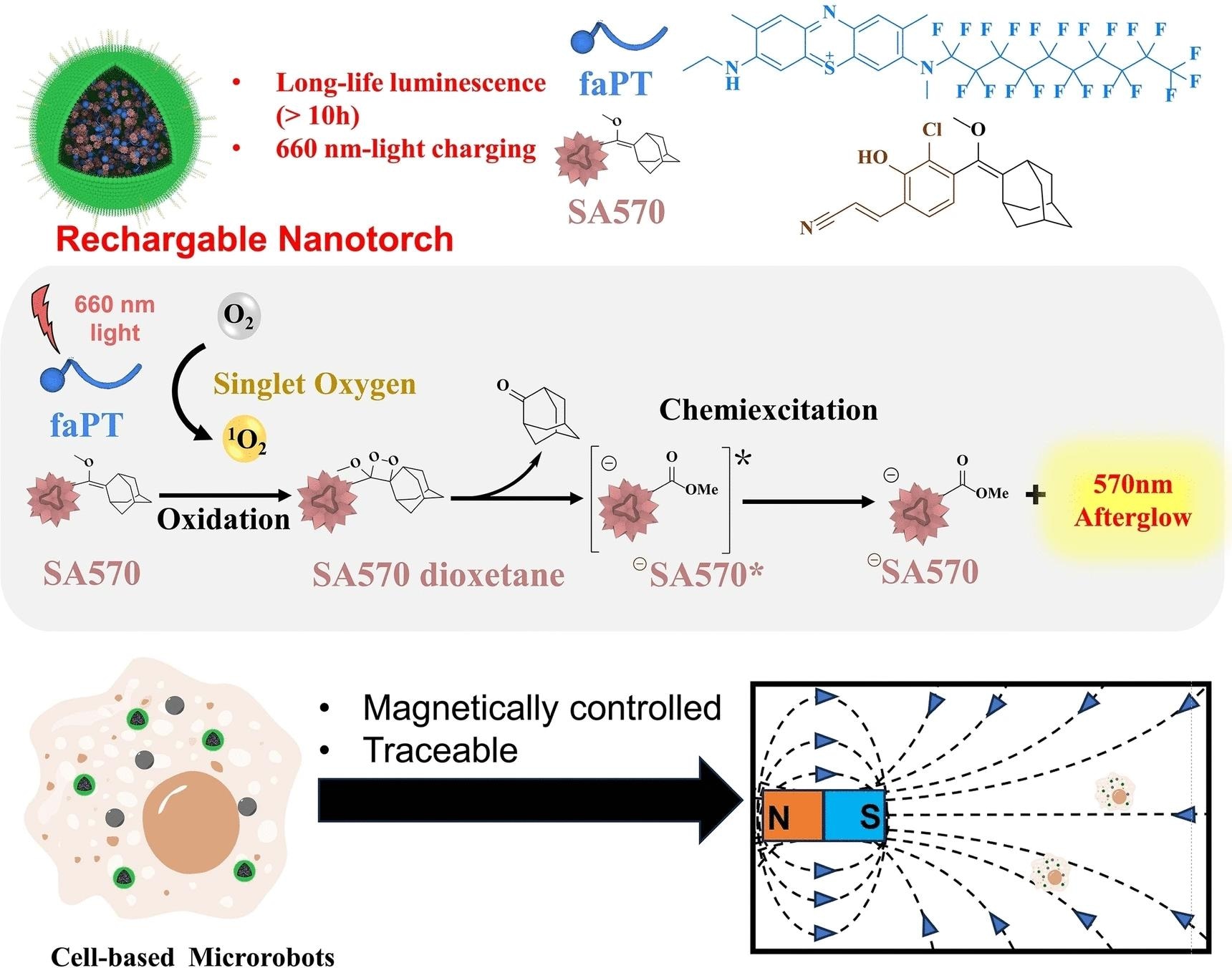
Image Credit: Wiley-VCH
Macrophages are crucial immune cells that “eat” bacteria and help dispose of cancer cells. They can also absorb drugs and transfer them into cells, including malignant cells. If macrophages absorb magnetic nanoparticles, they can be magnetized and directed to a specific place in the body, such as a tumor. This permits macrophage “microrobots” to decrease chemotherapy-related negative effects.
Following the microrobots over time as they go through the body would be beneficial. Fluorescence imaging approaches have been investigated; however, they need continuous external irradiation.
This results in a significant degree of background noise due to the autofluorescence of several biomolecules. Furthermore, the restricted penetration depth of visible and UV light into tissues restricts the detection depth.
One option could be to utilize probes that can be irradiated before the procedure to generate an afterglow. However, inorganic nanoparticles with long-lasting afterglow pose the risk of heavy metal ions leaking out, whereas organic compounds only luminesce briefly and cannot be repeatedly excited.
A team from the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences (China) has collaborated with Koç University (Turkey) to develop a “rechargeable nanotorch”.
It consists of many components, including nanoparticles of a precursor to a luminescent organic molecule, photosensitizers (a hydrophobic analog of methylene blue), and polyethylene glycol equipped with cell-penetrating peptides. The photosensitizer absorbs near-infrared light and stimulates the oxygen molecules around it.
This extremely reactive singlet oxygen then attaches to the precursor to produce a dioxetane group, a four-membered ring composed of two oxygen and two carbon atoms. This undergoes a rearrangement, releasing the required luminous molecule and emitting surplus energy via luminescence. After the first irradiation, the nanotorches continue to luminesce for 10 days.
Once depleted, the nanotorches can be “remotely” recharged using NIR light, which can repeatedly penetrate deeply into tissues to cause them to luminesce once again. To ensure that just some of the precursors are activated with each irradiation, the relative quantities of photosensitizer and luminous molecule precursor must be chosen. This makes longer time periods for imaging possible.
The China team, led by Pengfei Zhang, Ping Gong, and Lintao Cai, collaborated with the Turkey team, led by Safacan-Kolemen, to introduce these new nanotorches into macrophage-based microrobots and track their magnet-guided path through mice bodies in real time using luminescence signals.
Journal Reference:
Ma, G., et. al. (2024) Rechargeable Afterglow Nanotorches for In Vivo Tracing of Cell-Based Microrobots. Angewandte Chemie. doi:10.1002/anie.202400658