Reviewed by Lexie CornerJul 10 2024
In their most recent study published in the journal Physical Review Letters, researchers at the University of Basel investigated how the ferromagnetic characteristics of electrons in the two-dimensional semiconductor molybdenum disulfide may be better understood. They discovered a straightforward method for measuring the energy required to flip an electron spin.
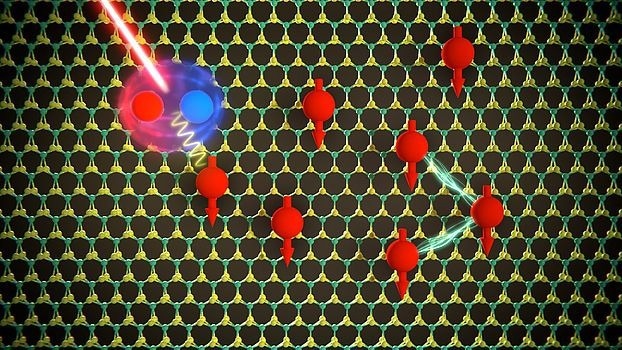 The two-dimensional semiconductor material molybdenum disulfide is filled with electrons (red spheres). The electron-electron interaction causes the spins of all electrons (red arrows) to align in the same direction. The exchange energy required to flip a single electron spin in the ferromagnetic state can be determined by the separation between two specific spectral lines. Image Credit: N. Leisgang, Harvard University, formerly Department of Physics, University of Basel/Scixel
The two-dimensional semiconductor material molybdenum disulfide is filled with electrons (red spheres). The electron-electron interaction causes the spins of all electrons (red arrows) to align in the same direction. The exchange energy required to flip a single electron spin in the ferromagnetic state can be determined by the separation between two specific spectral lines. Image Credit: N. Leisgang, Harvard University, formerly Department of Physics, University of Basel/Scixel
Ferromagnetism is an essential physical phenomenon used in various technologies. Metals such as iron, cobalt, and nickel are magnetic at normal temperatures because their electron spins are parallel; these materials lose their magnetic characteristics only at extremely high temperatures.
Researchers led by Professor Richard Warburton of the Department of Physics and the Swiss Nanoscience Institute at the University of Basel discovered that molybdenum disulfide has ferromagnetic characteristics under certain conditions. When treated to low temperatures and an external magnetic field, all of the electron spins in this material point the same way.
Researchers discovered how much energy is required to flip a single electron spin in this ferromagnetic condition. The term “exchange energy” is crucial since it explains the stability of ferromagnetism.
Detective Work Yielded a Simple Solution
We excited molybdenum disulfide using a laser and analyzed the spectral lines it emitted.
Dr. Nadine Leisgang, Study Main Author and Postdoctoral Fellow, Harvard University
Given that each spectral line corresponds to a given wavelength and energy, the researchers were able to calculate the exchange energy by measuring the distance between specific spectral lines. They discovered that in molybdenum disulfide, this energy is only around ten times smaller than in iron, indicating that the material's ferromagnetism is extremely stable.
Although the solution seems simple, it took considerable detective work to allocate the spectral lines correctly.
Richard Warburton, Professor, Department of Physics, University of Basel
Two-Dimensional Materials
2D materials play an important role in materials research due to their unique physical features caused by quantum mechanical phenomena. They can also be stacked to create “van der Waals heterostructures.”
This study's example shows a molybdenum disulfide layer surrounded by hexagonal boron nitride and graphene. Due to their distinct features, these layers, linked together by weak van der Waals connections, are of interest in electronics and optoelectronics. Understanding their electrical and optical characteristics is essential for applying them to future technologies.
Journal Reference:
Leisgang, N., et. al. (2024) Exchange Energy of the Ferromagnetic Electronic Ground State in a Monolayer Semiconductor. Physical Review Letters. doi:10.1103/PhysRevLett.133.026501