A Chinese research team illustrated in their study published in Carbon Future on June 21st, 2024, how a series of phosphorous-modified nanocarbon catalysts could improve green DMM manufacturing.
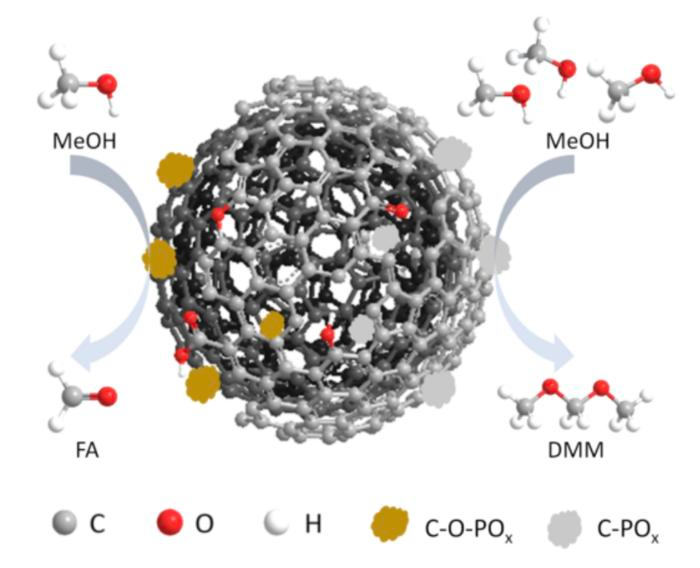 Achieving one step synthesis of DMM through phosphorous-modified nanocarbon catalyst. Image Credit: Wei Qi, the University of Science and Technology of China.
Achieving one step synthesis of DMM through phosphorous-modified nanocarbon catalyst. Image Credit: Wei Qi, the University of Science and Technology of China.
Vehicle exhaust from burning fossil fuels is a major source of air pollutants such as carbon dioxide and monoxide. To reduce air pollution, researchers are looking at fuel additives such as dimethoxymethane (DMM). However, DMM manufacturing has its own environmental risks.
This diesel mix fuel has unique fuel qualities, including a high oxygen concentration, chemical stability, and low toxicity. A combination of DMM with standard diesel fuel has been proven to minimize soot production by up to 80%.
DMM is commercially created using a two-step process that begins with methanol oxidation, which produces formaldehyde and then is coupled with methanol. However, because of the sophisticated sequencing reactions and the use of toxic acidic catalysts, this traditional synthesis approach is difficult and ecologically harmful.
To address these limitations, researchers have been investigating new techniques for producing DMM. In one potential technique, the use of nonmetallic nanocarbon materials as catalysts allows for the manufacture of DMM in a single step.
Non-metallic nanocarbon-based catalysts have arisen in recent years as long-lasting, dependable alternatives to metal catalysts, which have historically served as supports in chemical processes.
One-step synthesis of DMM via selective oxidation of methanol under the catalysis of nanocarbon is a green and sustainable but challenging chemical process. Nanocarbon materials have demonstrated notable activity and stability in various catalytic reactions.
Wei Qui, University of Science and Technology of China
However, completing one-step synthesis of DMM via methanol conversion necessitates striking a careful balance between redox (oxidation-reduction process) and acid sites, and many concerns remain unsolved about nano carbon catalysts.
For example, functional groups on the surface have a substantial impact on the performance of nanocarbon materials; nevertheless, nanocarbon materials have uncontrolled surface functional groups, making it difficult to identify active locations for various types of reactions.
Recent research has demonstrated that altering nanocarbons with nonmetallic heteroatoms may substantially alter surface properties and redox/acidic catalytic activity, resulting in extremely efficient and selective DMM synthesis pathways.
Expanding on this line of study, the Chinese researchers developed a variety of phosphorus-modified carbon catalysts for the one-step synthesis of DMM from methanol.
Using this method, the team was able to obtain high methanol conversion and DMM creation rates concurrently.
Their research revealed that the covalent linkage of phosphorus and nanocarbon (specifically, a bond where a carbon atom and a phosphorus atom share a pair of electrons) is a key factor contributing to high DMM selectivity, indicating efficiency and precision with a catalyst that converts raw materials into fuel additive products.
Qi added, “This work provided not only a novel and sustainable carbon-based catalyst for the one step synthesis of DMM but also deep insights into the rational design of nanocarbon catalyst for related reaction system.”
Their study proposed a new approach to the construction of innovative nanocarbon materials, as well as a possible green catalyst for the effective selective conversion of methanol to DMM.
The Natural Science Foundation of Liaoning Province, China Baowu Low Carbon Metallurgy Innovation Foundation, and the Shccig-Qinling program also funded the research.
Xueya Dai, Pengqiang Yan, Yunli Bai, and Miao Guo from the Institute of Metal Research at the Chinese Academy of Sciences. Dai and Bai are also associated with the University of Science and Technology of China as the other study contributors.
Journal Reference:
Dai, X., et. al. (2024) Phosphorus modified onion-like carbon catalyzed methanol conversion to dimethoxymethane: The unique role of C–P species. Carbon Future. doi:10.26599/CF.2024.9200012