Scientists at Johns Hopkins have created unique nanoparticles that can deliver gene therapy straight to different kinds of bone marrow cells, fixing the mutations that cause the disease. The complicated, drawn-out gene therapies used to treat sickle cell disease can occasionally have grave side effects like blood cancer or infertility. The study has been published in Nature Nanotechnology.
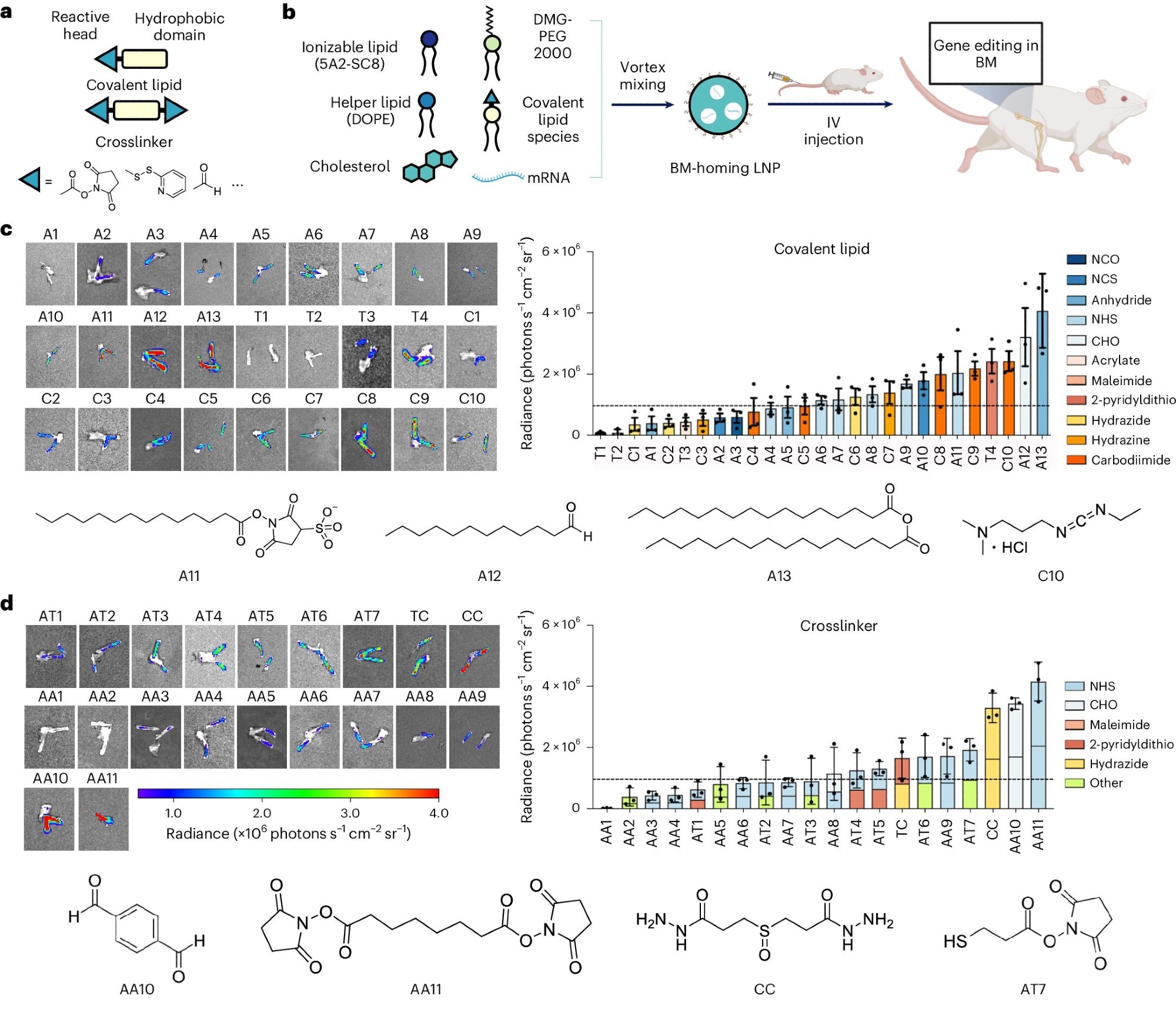 Discovery and development of BM-homing LNPs. a, Schematic of LNP preparation including covalent lipid species (covalent lipids and crosslinkers). b, Addition of a covalent lipid or crosslinker to the base-4-lipid LNP formulation leads to BM mRNA delivery and genome editing in a great breadth of unique BM cell types. c,d, Bioluminescence images of dissected femurs and summary of the average bioluminescence signal intensity on dissected femurs represented for covalent lipid (c) and crosslinker (d) molecular structures. Image Credit: Nature Nanotechnology.
Discovery and development of BM-homing LNPs. a, Schematic of LNP preparation including covalent lipid species (covalent lipids and crosslinkers). b, Addition of a covalent lipid or crosslinker to the base-4-lipid LNP formulation leads to BM mRNA delivery and genome editing in a great breadth of unique BM cell types. c,d, Bioluminescence images of dissected femurs and summary of the average bioluminescence signal intensity on dissected femurs represented for covalent lipid (c) and crosslinker (d) molecular structures. Image Credit: Nature Nanotechnology.
This gene editing approach would allow patients to receive the medicine through a transfusion. This avoids the lengthy, difficult process of many current gene therapies, decreasing the burden on patients and the health care system while minimizing treatment side effects.
Xizhen Lian, Study Lead Author and Assistant Research Scientist, Institute for Nano BioTechnology, Whiting School of Engineering
Xizhen Lian is also associated with the Johns Hopkins School of Medicine, Johns Hopkins University.
Utilizing CRISPR/Cas and base gene-editing techniques in a sickle cell disease mouse model, the research team—which included scientists from the University of Texas Southwestern Medical Center, St. Jude Children's Research Hospital, Harvard University, and Johns Hopkins School of Medicine—was able to correct the sickle cell mutation and activate a form of hemoglobin. Targeting leukemia cells was another area where the team found the approach effective.
One challenge we encountered is that the stem cell population is very small; only 0.1% of cells in bone marrow are stem cells. They are also protected in a micro-environment that can prevent the delivery of drugs from circulation.
Xizhen Lian, Study Lead Author and Assistant Research Scientist, Institute for Nano BioTechnology, Whiting School of Engineering
The team solved this issue by including a unique fat molecule in their tiny delivery particles. To deliver crucial gene therapy, this novel molecule assisted the delivery particles in locating and firmly attaching to the stem cells.
As they are now only using rodent blood cells and components, the team's next step is to optimize this technology on a humanized animal model that can more closely resemble clinical scenarios. By genetically modifying animals to express human genes, cells, and proteins, researchers can study human diseases in a living system very similar to humans: humanized animal models.
Lian said, “Our approach promises to help patients avoid invasive treatment procedures, which will significantly reduce the side effects of blood cancer because there is no random gene insertion into the patient's genes. We are targeting a specific gene that causes the disease and that's it. The only way to cure such genetic diseases is to correct the genetic mutation in the stem cell populations.”
Journal Reference:
Lian, X., et al. (2024) Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells. Nature Nanotechnology. doi.org/10.1038/s41565-024-01680-8