Researchers from Tokyo Metropolitan University developed sheets of transition metal chalcogenide “cubes” joined by chlorine atoms in a study published in Advanced Materials. The team's approach lays new ground by employing clusters instead of sheets of atoms, which have been extensively investigated in materials like graphene.
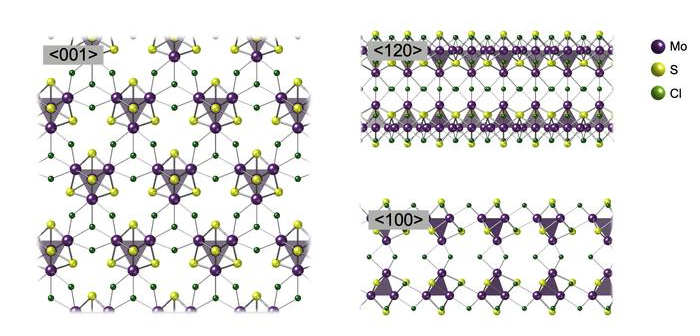 <001>, <120>, and <100> show the arrangement of the nanosheet when viewed from different directions, respectively. Image Credit: Tokyo Metropolitan University
<001>, <120>, and <100> show the arrangement of the nanosheet when viewed from different directions, respectively. Image Credit: Tokyo Metropolitan University
The researchers managed to generate microscale cube sheets that could be exfoliated and probed and nanoribbons inside carbon nanotubes for structural characterization. They demonstrated that they were very effective catalysts for producing hydrogen.
The development of two-dimensional materials, which have unique electronic and physical characteristics due to their sheet-like structure, is a significant advancement in nanotechnology.
Although graphene is a well-known material, transition metal chalcogenides (TMCs), made of a transition metal and a group 16 element such as selenium or sulfur, have also received much attention. TMC nanosheets, for instance, have demonstrated outstanding transistor performance and the ability to emit light.
Though tremendous progress is being made, most of it has been focused on getting atoms to form the proper crystalline structure in geometries resembling sheets.
Under the direction of Assistant Professor Yusuke Nakanishi, a group of researchers from Tokyo Metropolitan University were motivated to attempt an alternative strategy: can TMC clusters be used in place of TMC and arranged into two-dimensional patterns? This alternative method of creating nanosheets would result in an entirely new class of nanomaterials.
The group concentrated on cubic “superatomic” sulfur and molybdenum clusters. Using a vapor of sulfur and molybdenum (V) chloride, they created the material within the nanoscale constraints of carbon nanotubes.
Transmission electron microscopy (TEM) provides crisp images of the generated well-isolated nanoribbons. It was verified that their substance comprised separate molybdenum sulfide “cubes” joined by chlorine atoms instead of the cubic structures seen in bulk materials.
However, larger dimensions must be produced for the material to be functional in applications. The scientists discovered a flaky substance within their glass reaction tube during the same experiment. They found that the solid was constructed of relatively large microscale flakes composed of the same superatomic clusters organized in a hexagonal configuration by separating it from the walls.
While the researchers have only begun to investigate the possibilities of their novel material, they have demonstrated theoretically that the same structure under minuscule pressures could produce light. They also discovered that it could catalyze the hydrogen evolution process (HER), which occurs when a current travels through water and produces hydrogen.
Compared to molybdenum disulfide, a promising catalytic material, the novel layer produced much more current at lower voltages, indicating superior efficiency.
While there is more to come, their innovative method of assembling nanosheets offers a slew of new rationally designed materials with interesting new applications.
JSPS KAKENHI Grants from MEXT (Grant Numbers JP23H01807, JP24H00044, JP24K17708, JP24H01189, JP24H00478, JP22H05478, JP23H00277, JP21H05235, JP21K14484, JP21H05233, JP21H05232, JP21H05234, JP22H00283 and JP22H04957), and the PRESTO (Grant Number JPMJPR23H5), CREST (Grant Numbers JPMJCR20B1 and JPMJCR23A4), ACT-X (grant No. JPMJAX23DH), and FOREST (JPMJFR203K and JPMJFR213X) Programs from the JST supported the study.
Journal Reference:
Nakanishi, Y., et al. (2024) Superatomic Layer of Cubic Mo4S4 Clusters Connected by Cl Cross-Linking. Advanced Materials. doi:10.1002/adma.202404249