Reviewed by Danielle Ellis, B.Sc.Sep 20 2024
Groundwater is a vital supply of drinking water; on the other hand, groundwater contaminated by heavy metals poses a serious health risk. A solution based on nanomaterials has been developed by researchers from the Indian Institute of Science along with the researchers from the Centre for Sustainable Technologies, Department of Civil Engineering, and Department of Instrumentation and Applied Physics to efficiently minimize the level of heavy metals like chromium in groundwater. The Journal of Water Process Engineering published the study.
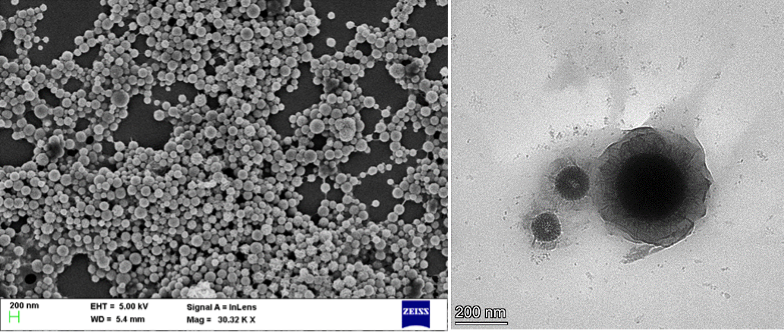 SEM and TEM images of S-CMC-nZVI. Image Credit: CeNSE, Indian Institute of Science
SEM and TEM images of S-CMC-nZVI. Image Credit: CeNSE, Indian Institute of Science
Usually, wastewater from businesses like textile production, electroplating, and leather tanning finds its way into soil and groundwater.
Heavy metals enter the environment because of urbanization and certain mismanagement by industries.
Prathima Basavaraju, Ph.D. Student and Study Lead Author, Centre for Sustainable Technologies
The majority of contemporary heavy metal contamination removal techniques include pumping water out of the ground and purifying it at a different location utilizing ion exchange, chemical precipitation, adsorption, and reverse osmosis. Instead, the IISc team suggests an on-site remedy that makes use of iron nanoparticles to remove heavy metals.
If the groundwater is contaminated, we can inject these nanoparticles into the subsurface groundwater region where it will react with the chromium and immobilize it, resulting in clear water.
Prathima Basavaraju, Ph.D. Student and Study Lead Author, Centre for Sustainable Technologies
Initially, the group attempted to synthesize nanoparticles made of nanozero-valent iron (nZVI). Co-precipitation can occur when this form of iron reacts with the poisonous and cancer-causing form of chromium (Cr6+) to convert it to the less dangerous form (Cr3+). Nevertheless, the group quickly discovered that the nZVI particles tended to group, which restricted their use.
The group used carboxymethyl cellulose (CMC) to stop clumping.
“We modified nZVI by coating it with CMC. It forms a stabilizing layer around nZVI separating individual particles,” Prathima explained.
The CMC covering also kept the iron core from oxidizing, extending the material's lifespan. Additionally, the group increased the CMC-nZVI's reactivity by subjecting it to sulfur-containing substances in anoxic environments.
This made it possible for the process known as sulfidation to occur, which produced a layer of protective iron sulfide on the surface. These adjustments preserved the S-CMC-nZVI's efficiency and reactivity while enhancing its stability.
When exposed to varying settings, such as varying pH levels and the presence of other competing ions that may be present in groundwater, S-CMC-nZVI demonstrated almost 99% efficacy at Cr6+ removal. The improved nanomaterial was put to the test in settings that closely resemble groundwater aquifers' natural habitat.
They saw strong remedial activity when they ran tainted water through sand columns that contained the nanomaterial. nZVI was also used in experiments to immobilize the heavy metals in contaminated soil and sediments. Experiments with scaling up are still ongoing.
S-CMC-nZVI is a material that shows promise for on-site cleanup of groundwater contaminated with chromium, according to the authors.
Places like Bellandur Lake [in Bengaluru] have a lot of contaminated sediments. The technique developed can also prove quite useful in remediating contaminants such as cadmium, nickel, and chromium in contaminated sediments of Bellandur Lake.
GL Sivakumar Babu, Professor, Study Co-Author, Centre for Sustainable Technologies
Journal Reference:
Prathima, B., et al.(2024) Sulfide-enhanced carboxymethyl cellulose stabilized nano zero-valent iron for chromium(VI) mitigation in water: Evidence from batch and column studies. Journal of Water Process Engineering. doi.org/10.1016/j.jwpe.2024.105832.