Reviewed by Lexie CornerOct 3 2024
Researchers from the University of California, San Diego have published a study in Advanced Functional Materials that could open up new possibilities in drug delivery and gene therapy, where genetic material needs to be delivered directly into the nucleus.
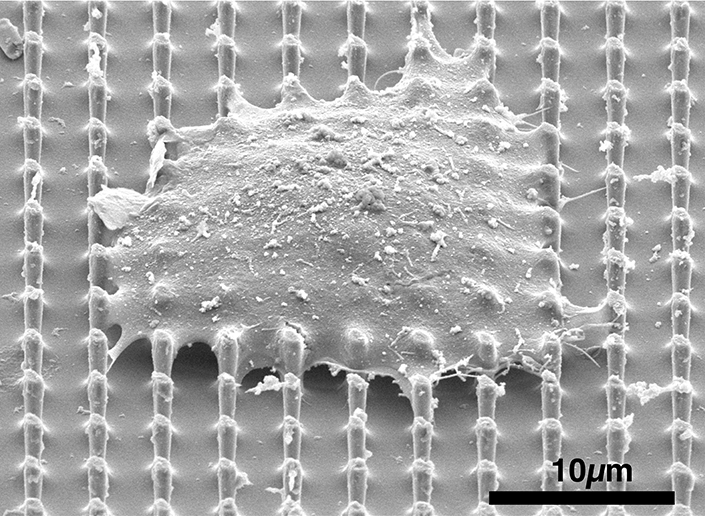 SEM image of a cell sitting on top of the nanopillar array. Image Credit: Ali Sarikhani
SEM image of a cell sitting on top of the nanopillar array. Image Credit: Ali Sarikhani
The researchers have developed a technology that performs a highly delicate task in living cells, comparable to puncturing a hole in a raw egg's yolk without breaking the surrounding egg white. They created specialized nanopillars capable of entering a cell's nucleus—where DNA is housed—without damaging the cell membrane.
We have developed a tool that can easily create a gateway into the nucleus.
Zeinab Jahed, Study Senior Author and Professor, Aiiso Yufeng Li Family Department of Chemical and Nano Engineering, University of California San Diego
The nucleus is designed to be impenetrable, and its membrane acts as a selective shield that permits only specific molecules to pass through controlled channels, thus protecting genetic material.
Jahed added, “It is not easy to get anything into the nucleus. Drug and gene delivery through the nuclear membrane has long been an immense challenge.”
Current methods to access the nucleus typically involve physically puncturing both the cell and nuclear membranes using tiny needles. These methods, however, are invasive and suited only for limited-scale applications.
To address this, Ali Sarikhani, a Ph.D. candidate in nanoengineering at UC San Diego, co-led a team to develop a non-disruptive alternative. They designed a series of nanopillars—small, cylindrical structures—that prompt the nuclear membrane to curve around them when a cell is placed on top. This curvature causes temporary, self-sealing openings in the nuclear membrane while leaving the cell's outer membrane unharmed.
“This is exciting because we can selectively create these tiny breaches in the nuclear membrane to access the nucleus directly while leaving the rest of the cell intact,” Jahed added.
In the experiments, cells with nuclei containing fluorescent dye were placed on the nanopillars. The dye was observed leaking from the nucleus into the cytoplasm but remained inside the cell, indicating that while the nuclear membrane had been temporarily disrupted, the cell membrane remained intact. This effect was noted across various cell types, including fibroblasts, heart muscle cells, and epithelial cells.
The researchers are currently investigating the mechanisms responsible for this effect.
Jahed added, “Understanding these details will be key to optimizing the platform for clinical use and ensuring that it is both safe and effective for delivering genetic material into the nucleus.”
The study's co-authors include Einollah Sarikhani, Vrund Patel, Zhi Li, Dhivya Pushpa Meganathan, Keivan Rahmani, Leah Sadr, Ryan Hosseini, Diether Visda, Shivani Shukla, Hamed Naghsh-Nilchi, Adarsh Balaji, Gillian McMahon, Shaoming Chen, Johannes Schöneberg, Colleen A. McHugh, and Lingyan Shi, all from UC San Diego.
The research received support from several funding sources, including the National Institutes of Health (R00 GM12049403, R01GM149976, and R21NS125395), the Air Force Office of Scientific Research YIP award (311616-00001), a faculty seed grant from the Cancer Research Coordinating Committee, and startup funds from UC San Diego. Part of the study was conducted at the San Diego Nanotechnology Infrastructure (SDNI) at UC San Diego, a member of the National Nanotechnology Coordinated Infrastructure, funded by the National Science Foundation (grant ECCS-2025752).
Journal Reference:
Sarikhani, E. (2024) Engineered Nanotopographies Induce Transient Openings in the Nuclear Membrane. Advanced Functional Materials. doi.org/10.1002/adfm.202410035