Reviewed by Lexie CornerNov 14 2024
An international team of scientists, led by researchers from the Institute of Nuclear Physics of the Polish Academy of Sciences (IFJ PAN) in Krakow, conducted an advanced experiment to demonstrate the electrodeposition process of a platinum-nickel (PtNi) nanolayer on an electrode. This research was published in Nano Letters.
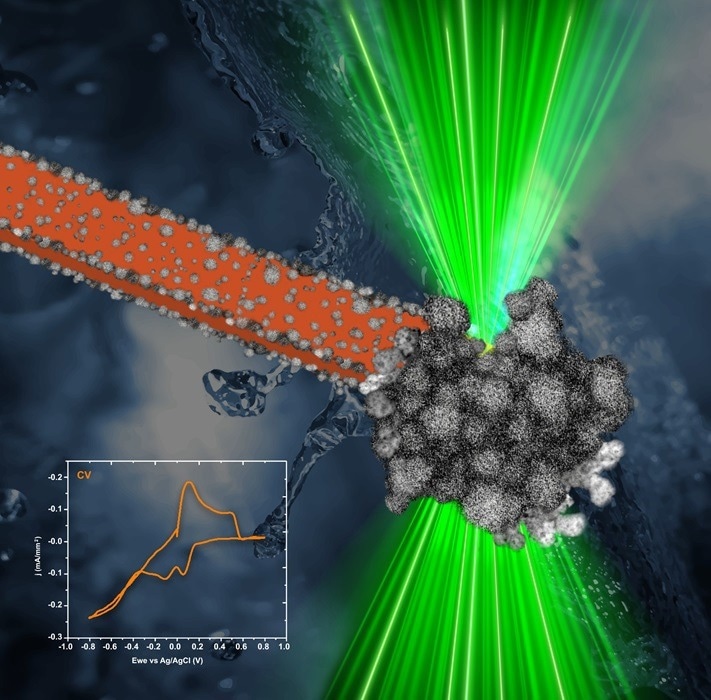 In-situ liquid-cell transmission electron microscopy electrodeposition of PtNi nanoparticle films on a carbon electrode during cyclic voltammetry. The electron beam (here in green color) illuminated the electrode (here in orange color) submerged in the platinum and nickel salt solution, enhancing the growth of the PtNi nanoparticle film (grey color) on the electrode. The film thickness increases with each cycle, and by the fourth cycle, reaction-rate limited growth of branched and porous structures was observed. Image Credit: Weronika Wojtowicz
In-situ liquid-cell transmission electron microscopy electrodeposition of PtNi nanoparticle films on a carbon electrode during cyclic voltammetry. The electron beam (here in green color) illuminated the electrode (here in orange color) submerged in the platinum and nickel salt solution, enhancing the growth of the PtNi nanoparticle film (grey color) on the electrode. The film thickness increases with each cycle, and by the fourth cycle, reaction-rate limited growth of branched and porous structures was observed. Image Credit: Weronika Wojtowicz
Metallic nanoparticles, ranging from a few to several thousand atoms or simple molecules, are gaining attention due to their potential applications. Electrodes coated with nanoparticle layers (nanolayers) are useful as catalysts in energy production.
Electrodeposition is a practical method for creating these layers on electrodes. An international team of researchers, led by experts from the Institute of Nuclear Physics at the Polish Academy of Sciences in Krakow, has explored the complexities of this process.
Nanoparticle research is providing valuable insights for energy, medical, and electronics applications. However, controlling the creation and growth of nanostructures remains a significant challenge. The team demonstrated the electrodeposition of a PtNi nanolayer on an electrode.
Using advanced imaging tools, the researchers were able to observe, in real-time, how the structures grow at the atomic level, providing important insights for engineering materials with precisely controlled properties.
Electrodeposition is a straightforward method for creating nanostructures. It involves immersing an electrode in a metal salt solution, followed by applying a sufficient voltage to reduce the ions near the electrode surface, initiating layer formation. To analyze the electrodeposition process in detail, transmission electron microscopy (TEM) is commonly used.
TEM uses an electron beam with a wavelength much shorter than visible light, allowing for imaging at sub-angstrom resolution, or less than one ten-millionth of a millimeter. Ideally, it would be possible to observe in real-time how the layer grows and how nucleation—the initial stage of growth where nanoparticle seeds form—occurs on the electrode.
However, there are limitations with TEM imaging: the samples must be completely dry and as thin as possible. To overcome these challenges, the researchers used a unique imaging technique within a liquid cell flow chamber, enabling the observation of chemical reactions.
The flow cell consists of two silicon chips equipped with a 50 nm thick SiNx membrane. This membrane is electron-transparent, and an additional electrode is placed on its surface. By applying a voltage, the microscope user can observe how the layer grows on the electrode. Experiments using such a cell require a special holder for flow experiments in the TEM.
Magdalena Parlińska-Wojtan, PhD, Professor, Institute of Nuclear Physics
The PtNi layer forms directly on the electrode, as confirmed by experiments conducted at the Silesian University of Technology using a TEM microscope. These experiments offered valuable insights into the underlying process. One possibility is that nanoparticles initially form in the electrolyte and then migrate toward the electrode, where they attach.
This effect was also observed, but it was limited to areas illuminated by the electron beam. The interaction of the electron beam with water, which serves as a reduction agent, influenced the process. Further analysis in "dry" conditions revealed that the layer consists of spherical nanoparticles, each with sizes ranging from several tens of nanometers. TEM images taken at higher magnifications showed that the surfaces of these nanoparticles are covered with fine, densely branched dendritic structures.
Parlińska-Wojtan added, “As part of our collaboration with the Fritz Haber Institute of the Max Planck Society in Berlin, we conducted an additional experiment by extending the reaction time and reducing the rate of voltage changes. This allowed us to observe additional effects: the nucleation of individual nanoparticles, which rapidly grow and merge to form a continuous layer. During voltage changes in subsequent electrodeposition cycles, the nanoparticles undergo alternating growth and dissolution. However, growth is a faster process than dissolution, which ultimately results in a stable layer.”
Another experiment was conducted in a liquid environment using a scanning transmission X-ray microscope (STXM) at the National Synchrotron Radiation Center SOLARIS in Krakow. In STXM imaging, X-ray radiation is used to capture images.
While the resolution of STXM images is lower than that of electron microscopy, this technique provides valuable information about the materials being studied, such as the oxidation states of atoms in the nanoparticles. Electrodeposition does not always result in pure metal; it can also produce metal oxides.
Different materials absorb X-ray radiation at varying energies depending on whether they are metals or oxides, as well as the oxidation state of the oxide. By using the appropriate energy beam, STXM images allow for detailed analysis of the nanoparticles. In this experiment, the PtNi electrodes were placed in the STXM and analyzed in real-time to observe the X-ray absorption characteristics. The results revealed that the layer consisted of nickel(II) oxide and metallic platinum.
“Conducting an experiment using microscopic techniques in a liquid environment is quite a challenge. Nevertheless, our team succeeded in producing the expected PtNi layer using two different techniques, and the obtained results were complementary,” Parlińska-Wojtan stated.
Parlińska-Wojtan, emphasized, “Such research is important for several reasons. The technical reason is that we are still exploring the capabilities and limitations of relatively new, high-end measurement tools. There was also a more important scientific reason: understanding the fundamental factors that govern the synthesis, growth, and properties of nanostructures. This knowledge may help in the future in the fabrication of nanostructured materials tailored better for applications such as fuel cells or medicine.”
Journal Reference:
Parlinska-Wojtan, M ., et al. (2024) Understanding the Growth of Electrodeposited PtNi Nanoparticle Films Using Correlated In Situ Liquid Cell Transmission Electron Microscopy and Synchrotron Radiation. Nano Letters. doi.org/10.1021/acs.nanolett.4c02228.