An international group of Scientists from Tohoku University, Tokyo University of Science, and the University of Adelaide have developed a novel technique to improve the sustainability and selectivity of electrochemical CO2 reduction processes. The findings were published in the journal Small.
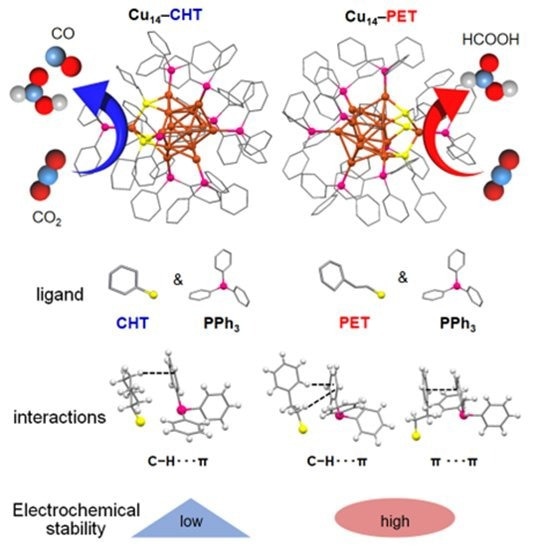 Detailed architecture of two distinct Cu14 NCs, protected by two different thiols which were investigated in this study. These NCs exhibit different intercluster interactions which shape their stability and reaction selectivity for electrochemical CO2 reduction reaction. Image Credit: Yuichi Negishi et al.
Detailed architecture of two distinct Cu14 NCs, protected by two different thiols which were investigated in this study. These NCs exhibit different intercluster interactions which shape their stability and reaction selectivity for electrochemical CO2 reduction reaction. Image Credit: Yuichi Negishi et al.
While copper (Cu) may not be as appealing as gold or silver, its incredible adaptability makes it vital in cutting-edge research.
Through atomic-level surface engineering of Cu nanoclusters (NCs), the team has opened up new avenues for effective and environmentally responsible carbon conversion technologies. This discovery demonstrates the revolutionary potential of copper in sustainable chemistry and emphasizes the importance of international cooperation in tackling urgent issues like carbon emissions.
Electrochemical CO2 reduction reactions (CO2RR) have received considerable attention in recent years due to their ability to convert surplus atmospheric CO2 into valuable products. Among the different nanocatalysts examined, NCs have stood out due to their particular benefits over bigger nanoparticles. Within this class, Cu NCs have shown considerable potential, allowing for the synthesis of varied products, strong catalytic activity, and sustainability.
Despite these advantages, achieving exact control over product selectivity on an industrial scale remains difficult. As a result, current research is heavily focused on improving these qualities to realize the full potential of Cu NCs for sustainable CO2 conversion.
To achieve this breakthrough, our team had to modify NCs at the atomic scale. However, it is very challenging since the geometry of the NCs was heavily dependent on the precise parts that we needed to alter. It was like trying to move a supporting pillar of a building.
Yuichi Negishi, Professor, Tohoku University
The researchers successfully produced two Cu₁₄ NCs with identical structural architectures by changing the thiolate ligands (PET: 2-phenylethanethiolate; CHT: cyclohexanethiolate) on the surfaces. Overcoming this limitation necessitated the invention of a carefully regulated reduction method, which allowed for the formation of two structurally similar NCs with unique ligands--a significant advancement in NC design.
The scientists did, however, discover variations in the stability of these NCs, which they attributed to changes in intercluster interactions. These differences significantly impact the long-term viability of these NCs in catalytic applications.
Although these NCs have similar geometries obtained from two different thiolate ligands, they show drastically different product selectivity when their catalytic activity for CO2 reduction is examined. These changes affect the CO2RR’s overall efficiency and selectivity.
Negishi concluded, “These findings are pivotal for advancing the design of Cu NCs that combine stability with high selectivity, paving the way for more efficient and reliable electrochemical CO2 reduction technologies.”
Journal Reference:
Shingyouchi, Y., et al. (2024) Ligand‐Dependent Intracluster Interactions in Electrochemical CO2 Reduction Using Cu14 Nanoclusters. Small. https://doi.org/10.1002/smll.202409910.