Reviewed by Lexie CornerJan 16 2025
Chemists from Würzburg have developed a novel model system for selective ion transport. The system, composed of a stable double layer with a nanographene-confined cavity, demonstrates high selectivity by allowing the transport of fluoride, chloride, and bromide ions while effectively excluding iodide ions. The findings were published in Nature.
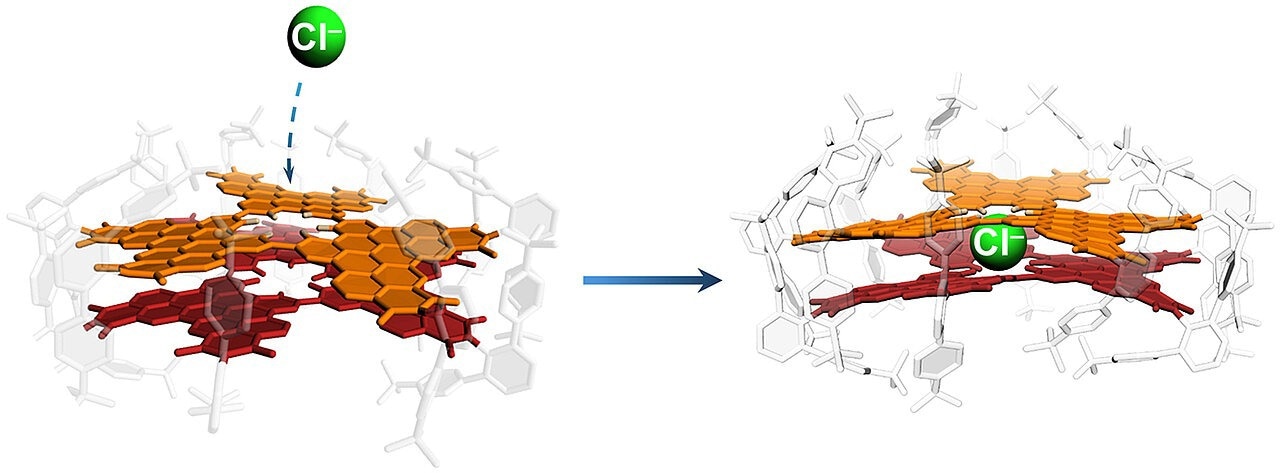 The Würzburg model system consisting of two nanographene layers that can absorb and bind chloride ions (green) through a defect in the crystal lattice. Image Credit: Kazutaka Shoyama / Universität Würzburg
The Würzburg model system consisting of two nanographene layers that can absorb and bind chloride ions (green) through a defect in the crystal lattice. Image Credit: Kazutaka Shoyama / Universität Würzburg
Graphene, a material composed of pure carbon, is exceptionally thin, flexible, and durable. It forms layers consisting of nearly a single layer of carbon atoms each. To achieve a thickness equivalent to a human hair, thousands of these layers would need to be stacked.
Graphene continues to be a major focus of research due to its unique properties, which hold promise for innovative applications in fields such as energy and electronics.
Making Graphene Permeable to Other Molecules
The ability to control graphene's permeability to different substances is of particular interest to scientists.
So-called defects can be created in the carbon lattice of graphene. These can be thought of as small holes that make the lattice permeable to gases.
Frank Würthner, Professor, Chemistry, Julius-Maximilians-Universität Würzburg
There is currently no evidence of permeability to other substances, such as ions like fluoride, chloride, or bromide.
However, this would be of fundamental scientific interest for applications such as the desalination of water, the detection or purification of mixtures of substances.
Frank Würthner, Professor, Chemistry, Julius-Maximilians-Universität Würzburg
Defect Allows Ions to Pass Through: Publication in Nature
For the first time, a team led by Frank Würthner has developed a model system with a defect that selectively permits the passage of fluoride, chloride, and bromide ions while excluding iodide. This was accomplished using a stable double layer of two nanographenes that form a cavity. The halide ions that penetrate are bound within the cavity, allowing the time required for their entry to be measured.
Chloride, a component of common salt and a natural constituent of seawater, is essential for life processes in all organisms.
The proof of a high permeability for chloride by single-layer nanographene and a selective binding of halides in a double-layer nanographene brings some applications closer.
Dr. Kazutaka Shoyama, Study Corresponding Author, Institut für Organische Chemie, Universität Würzburg
Chloride channels, synthetic receptors, and water filtration membranes are among the potential applications for this research.
Larger Stacks of Nanographenes are the Next Goal
The Würzburg chemists aim to construct larger stacks of nanographenes in the next phase of their work. These will be used to study ion flow, a process analogous to that observed in biological ion channels.
This research was conducted at JMU's Center for Nanosystems Chemistry and Institute of Organic Chemistry, with support from two German Research Foundation (DFG) grants for the development of nanographenes with imide groups.
Journal Reference:
Würthner, F., et al. (2024) Bilayer nanographene reveals halide permeation through a benzene hole. Nature. doi.org/10.1038/s41586-024-08299-8