Reviewed by Lexie CornerMar 28 2025
Researchers from the Universities of Amsterdam and Zurich have collaborated to create a novel molecular system that enables the controlled release of iron. This breakthrough was achieved by combining ferrocene, a molecular structure that encapsulates an iron atom, with a carbon-based nanohoop.
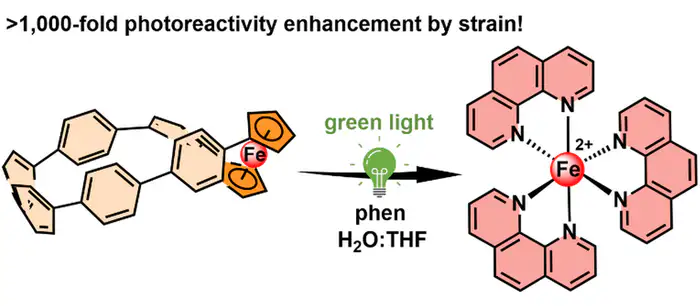
Image Credit: HIMS/Journal of the American Chemical Society
The resulting system facilitates the release of Fe2+ ions when activated by green light. According to the study, this approach has considerable potential.
The research was conducted by Dr. Tomáš Šolomek’s group at the University of Amsterdam’s Van 't Hoff Institute for Molecular Sciences and Dr. Peter Štacko’s group at the University of Zurich’s Department of Chemistry.
Their expertise lies in photocages—molecular photochemical tools that enable precise control over substrate activity in both time and space using light as a bio-orthogonal stimulus. Photocages allow the activation of biologically relevant molecules, including proteins, nucleotides, and drugs. Beyond their utility in studying biochemical mechanisms and dynamics, they also hold promise for therapeutic applications such as photoactivated chemotherapy.
In their study, the researchers moved beyond controlling the activity of organic molecules to focus on another essential element in biological systems: iron. Widely recognized for its role in oxygen transport in the human body, iron also plays a crucial part in energy-producing redox processes in mitochondria, deoxyribonucleotide synthesis, and cellular protection against oxidative stress.
Strain-Induced Photorelease
Nature has developed a protein-based system to tightly regulate iron’s uptake and balance. Inspired by this, the researchers engineered a synthetic system that stores and releases iron as needed.
The system uses ferrocene as the iron carrier, which is controlled by incorporating it into a carbon nanohoop. Ferrocene is an organometallic complex with an iron atom positioned between two cyclopentadienyl rings.
Although ferrocene itself is chemically stable and resistant to light, its integration into a molecular nanohoop changes these properties. Specifically, when incorporated into a cycloparaphenylene nanohoop—where the cyclopentadienyl rings are connected by six benzene rings—the system gains the ability to regulate iron containment. This integration induces a twist in the nanohoop structure, creating mechanical stress on the ferrocene, making the system sensitive to green light, which triggers the release of iron.
In their study, the researchers demonstrate that iron can be efficiently released upon irradiation. They anticipate that this approach, which leverages mechanical stress in molecules, could have significant applications beyond photocages. It may lead to the development of new responsive materials in supramolecular, organometallic, and polymer chemistry.
Journal Reference:
Kręcijasz, B. R., et al. (2025) Strain-Induced Photochemical Opening of Ferrocene[6]cycloparaphenylene: Uncaging of Fe2+ with Green Light. Journal of the American Chemical Society (JACS). doi.org/10.1021/jacs.4c15818